Foam

A foam is a substance that is formed by trapping pockets of gas in a liquid or solid.[1] A bath sponge and the head on a glass of beer are examples of foams. In most foams, the volume of gas is large, with thin films of liquid or solid separating the regions of gas.
An important division of solid foams is into closed-cell foams and open-cell foams. In a closed-cell foam, the gas forms discrete pockets, each completely surrounded by the solid material. In an open-cell foam, the gas pockets connect with each other. A bath sponge is an example of an open-cell foam: water can easily flow through the entire structure, displacing the air. A camping mat is an example of a closed-cell foam: the gas pockets are sealed from each other so the mat cannot soak up water.
Foams are examples of dispersed media. In general, gas is present in large amount so it will be divided into gas bubbles of many different sizes (the material is polydisperse) separated by liquid regions which may form films, thinner and thinner when the liquid phase is drained out of the system films.[2] When the principal scale is small, i.e. for a very fine foam, this dispersed medium can be considered as a type of colloid.
The term foam may also refer to anything that is analogous to such a foam, such as quantum foam, polyurethane foam (foam rubber), XPS foam, polystyrene, phenolic, or many other manufactured foams.
Structure

A foam is in many cases a multiscale system.

One scale is the bubble: material foams are typically disordered and have a variety of bubble sizes. At larger sizes, the study of idealized foams is closely linked to the mathematical problems of minimal surfaces and three-dimensional tessellations, also called honeycombs. The Weaire-Phelan structure is believed to be the best possible (optimal) unit cell of a perfectly ordered foam,[3] while Plateau's laws describe how soap-films form structures in foams.
At lower scale than the bubble is the thickness of the film for metastable foams, which can be considered as a network of interconnected films called lamellae. Ideally, the lamellae are connected in triads and radiate 120° outward from the connection points, known as Plateau borders.
An even lower scale is the liquid–air interface at the surface of the film. Most of the time this interface is stabilized by a layer of amphiphilic structure, often made of surfactants, particles (Pickering emulsion), or more complex associations.
Formation
Several conditions are needed to produce foam: there must be mechanical work, surface active components (surfactants) that reduce the surface tension, and the formation of foam faster than its breakdown. To create foam, work (W) is needed to increase the surface area (ΔA):
where γ is the surface tension.
One of the ways foam is created is through dispersion, where a large amount of gas is mixed with a liquid. A more specific method of dispersion involves injecting a gas through a hole in a solid into a liquid. If this process is completed very slowly, then one bubble can be emitted from the orifice at a time as shown in the picture below.
One of the theories put forth for determining the separation time is shown below; however, while this theory produces the theoretical data that matches with experimental data, detachment due to capillarity is accepted as a better explanation.

The buoyancy force will act to raise the bubble, which is
where 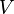 is the volume of the bubble,
is the volume of the bubble,  is the acceleration due to gravity, and ρ1 is the density of the gas ρ2 is the density of the liquid. The force working against the buoyancy force is the surface tension force, which is
is the acceleration due to gravity, and ρ1 is the density of the gas ρ2 is the density of the liquid. The force working against the buoyancy force is the surface tension force, which is
 ,
,
where γ is the surface tension, and  is the radius of the orifice.
As more air is pushed into the bubble, the buoyancy force grows quicker than the surface tension force. Thus, detachment will occur when the buoyancy force is large enough to overcome the surface tension force.
is the radius of the orifice.
As more air is pushed into the bubble, the buoyancy force grows quicker than the surface tension force. Thus, detachment will occur when the buoyancy force is large enough to overcome the surface tension force.
In addition, if the bubble is treated as a sphere with a radius of 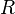 and the volume
and the volume 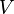 is substituted in to the equation above, separation occurs at the moment when
is substituted in to the equation above, separation occurs at the moment when
Examining this phenomenon from a capillarity viewpoint for a bubble that is being formed very slowly, it can be assumed that the pressure 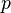 inside is constant everywhere. The hydrostatic pressure in the liquid is designated by
inside is constant everywhere. The hydrostatic pressure in the liquid is designated by  . The change in pressure across the interface from gas to liquid is equal to the capillary pressure; hence,
. The change in pressure across the interface from gas to liquid is equal to the capillary pressure; hence,
where R1 and R2 are the radii of curvature and are set as positive. At the stem of the bubble, R3 and R4 are the radii of curvature also treated as positive. Here the hydrostatic pressure in the liquid has to take in account z, the distance from the top to the stem of the bubble. The new hydrostatic pressure at the stem of the bubble is p0(ρ1-ρ2)z. The hydrostatic pressure balances the capillary pressure which is shown below:
Finally, the difference in the top and bottom pressure will equal the change in hydrostatic pressure:
At the stem of the bubble, the shape of the bubble is nearly cylindrical; consequently, either R3 or R4 will be very large while the other radius of curvature will be very small. As the stem of the bubble grows in length, it becomes more unstable as one of the radius grows and the other shrinks. At a certain point, the vertical length of the stem exceeds the circumference of the stem and due to the buoyancy forces the bubble separates and the process repeats.[4]
Stability
Stabilization

.png)
Stabilization of foam is caused by van der Waals forces between the molecules in the foam, electrical double layers created by dipolar surfactants, and the Marangoni effect, which acts as a restoring force to the lamellae.
The Marangoni effect is dependent on the liquid that is foaming usually not being pure. Generally, there are surfactants in the solution which will decrease the surface tension in the liquid. The surfactants will also clump together on the surface and form a layer as shown in the picture below.
For the Marangoni effect to occur, first the foam must be indented as shown in the first picture. This indentation will increase the local surface area. The surfactants have a larger diffusion time than the bulk of the solution; therefore, there is a smaller concentration of the surfactants in the indentation.
In addition, due to the stretching of the surface, the surface tension of the indented spot is greater than the surrounding area. Consequentially, since diffusion time for the surfactants is large, the Marangoni effect has time to take place. The difference in surface tension creates a gradient, which instigates fluid flow from areas of lower surface tension to areas of higher surface tension. The second picture shows the film at equilibrium after the Marangoni effect has taken place.[5]
Destabilization
Rybczynski and Hadamar developed an equation to calculate the velocity of bubbles that rise in foam with the assumption that the bubbles are spherical with a radius  .
.
with velocity in units of centimeters per second. ρ1 and ρ2 is the density for a gas and liquid respectively in units of g/cm3 and ῃ1 and ῃ2 is the viscosity of the gas and liquid g/cm·s and g is the acceleration in units of cm/s2.
However, since the density and viscosity of a liquid is much greater than the gas, the density and viscosity of the gas can be neglected which yields the new equation for velocity of bubbles rising as:
However, through experiments it has been shown that a more accurate model for bubbles rising is:
Reasons for the deviations are due to the Marangoni effect and capillary pressure which affects the assumption that the bubbles are spherical. For laplace pressure of a curved gas liquid interface, the two principle radii of curvature at a point are R1 and R2.[6] With a curved interface, the pressure in one phase will be greater than the pressure in another phase; the capillary pressure Pc is given by the equation of:
 ,
,
where 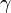 is the surface tension. The bubble shown below is a gas (phase 1) in a liquid (phase 2) and point A designates the top of the bubble while point B designates the bottom of the bubble.
is the surface tension. The bubble shown below is a gas (phase 1) in a liquid (phase 2) and point A designates the top of the bubble while point B designates the bottom of the bubble.

At the top of the bubble at point A, the pressure in the liquid is assumed to be p0 as well as in the gas. At the bottom of the bubble at point B, the hydrostatic pressure is:
where ρ1 and ρ2 is the density for a gas and liquid respectively. The difference in hydrostatic pressure at the top of the bubble is 0, while the difference in hydrostatic pressure at the bottom of the bubble across the interface is gz(ρ2 - ρ1). Assuming the that the radii of curvature at point A are equal and denoted by RA and that the that the radii of curvature at point B are equal and denoted by RB, then the difference in capillary pressure between point A and point B is:
At equilibrium, the difference in capillary pressure must be balanced by the difference in hydrostatic pressure. Hence,
Since, the density of the gas is less than the density of the liquid the left hand side of the equation will always be positive. Therefore, the inverse of RA must be larger than the RB. Meaning that from the top of the bubble to the bottom of the bubble the radius of curvature will increase; therefore, without neglecting gravity the bubbles cannot be spherical. In addition, as z increases, this will cause the difference in RA and RB too, which means the bubble will deviate more from its shape the larger it grows.[7]
Foam destabilization occurs for several reasons. First, gravitation causes drainage of liquid to the foam base, which Rybczynski and Hadamar include in their theory; however, foam also destabilizes due to osmotic pressure causes drainage from the lamellas to the Plateau borders due to internal concentration differences in the foam, and Laplace pressure causes diffusion of gas from small to large bubbles due to pressure difference. In addition, films can break under disjoining pressure, These effects can lead to rearrangement of the foam structure at scales larger than the bubbles, which may be individual (T1 process) or collective (even of the "avalanche" type).
Experiments and characterizations
Being a multiscale system involving many phenomena, and a versatile medium, foam can be studied using many different techniques. Considering the different scales, experimental techniques are diffraction ones, mainly light scattering techniques (DWS, see below, static and dynamic light scattering, X rays and neutron scattering) at sub-micrometer scales, or microscopic ones. Considering the system as continuous, its bulk properties can be characterized by light transmittance but also conductimetry. The correlation between structure and bulk is evidenced more accurately by acoustics in particular. The organisation between bubbles has been studied numerically using sequential attempts of evolution of the minimum surface energy either at random (Pott's model) or deterministic way (surface evolver). The evolution with time, i.e. the dynamics, can be simulated using these models, but also the bubble model (Durian) which considers the motion of individual bubbles.
Among possible examples, low scale observations of the structure done using reflectivity by the films between bubbles, of radiation, ponctual using laser or X rays beams, or more global using neutron scattering.


A typical light scattering (or diffusion) optical technique, multiple light scattering coupled with vertical scanning, is the most widely used technique to monitor the dispersion state of a product, hence identifying and quantifying destabilization phenomena.[8][9][10][11] It works on any concentrated dispersions without dilution, including foams. When light is sent through the sample, it is backscattered by the bubbles. The backscattering intensity is directly proportional to the size and volume fraction of the dispersed phase. Therefore, local changes in concentration (drainage, syneresis) and global changes in size (ripening, coalescence) are detected and monitored.
Applications
Liquid foams
Liquid foams can be used in fire retardant foam, such as those that are used in extinguishing fires, especially oil fires.
In some ways, leavened bread is a foam, as the yeast causes the bread to rise by producing tiny bubbles of gas in the dough. The dough has traditionally been understood as a closed-cell foam, in which the pores do not connect with each other. Cutting the dough releases the gas in the bubbles that are cut, but the gas in the rest of the dough cannot escape. When dough is allowed to rise too far, it becomes an open-cell foam, in which the gas pockets are connected. Now, if the dough is cut or the surface otherwise broken, a large volume of gas can escape, and the dough collapses. The open structure of an over-risen dough is easy to observe: instead of consisting of discrete gas bubbles, the dough consists of a gas space filled with threads of the flour-water paste. Recent research has indicated that the pore structure in bread is 99% interconnected into one large vacuole, thus the closed-cell foam of the moist dough is transformed into an open cell solid foam in the bread.[12]
The unique property of gas-liquid foams having very high specific surface area is exploited in the chemical processes of froth flotation and foam fractionation.
Solid foams
Solid foams are an important class of lightweight cellular engineering materials. These foams can be classified into two types based on their pore structure: open-cell-structured foams (also known as reticulated foams) and closed-cell foams.
Open-cell-structured foams contain pores that are connected to each other and form an interconnected network that is relatively soft. Open-cell foams will fill with whatever they are surrounded with. If filled with air, a relatively good insulator is the result, but, if the open cells fill with water, insulation properties would be reduced. Foam rubber is a type of open-cell foam.
Closed-cell foams do not have interconnected pores. The closed-cell foams normally have higher compressive strength due to their structures. However, closed-cell foams are also in general denser, require more material, and as a consequence are more expensive to produce. The closed cells can be filled with a specialized gas to provide improved insulation. The closed-cell structure foams have higher dimensional stability, low moisture absorption coefficients, and higher strength compared to open-cell-structured foams. All types of foam are widely used as core material in sandwich-structured composite materials.
From the early 20th century, various types of specially manufactured solid foams came into use. The low density of these foams makes them excellent as thermal insulators and flotation devices, and their lightness and compressibility makes them ideal as packing materials and stuffings.
Syntactic foam
A special class of closed-cell foams, known as syntactic foam, contains hollow particles embedded in a matrix material. The spheres can be made from several materials, including glass, ceramic, and polymers. The advantage of syntactic foams is that they have a very high strength-to-weight ratio, making them ideal materials for many applications, including deep-sea and space applications. One particular syntactic foam employs shape memory polymer as its matrix, enabling the foam to take on the characteristics of shape memory resins and composite materials; i.e., it has the ability to be reshaped repeatedly when heated above a certain temperature and cooled. Shape memory foams have many possible applications, such as dynamic structural support, flexible foam core, and expandable foam fill.
Integral skin foam
Integral skin foam, also known as self-skin foam, is a type of foam with a high-density skin and a low-density core. It can be formed in an open-mold process or a closed-mold process. In the open-mold process, two reactive components are mixed and poured into an open mold. The mold is then closed and the mixture is allowed to expand and cure. Examples of items produced using this process include arm rests, baby seats, shoe soles, and mattresses. The closed-mold process, more commonly known as reaction injection molding (RIM), injects the mixed components into a closed mold under high pressures.[13]
Defoaming
Foam, in this case meaning "bubbly liquid", is also produced as an often-unwanted by-product in the manufacture of various substances. For example, foam is a serious problem in the chemical industry, especially for biochemical processes. Many biological substances, for example proteins, easily create foam on agitation or aeration. Foam is a problem because it alters the liquid flow and blocks oxygen transfer from air (thereby preventing microbial respiration in aerobic fermentation processes). For this reason, anti-foaming agents, like silicone oils, are added to prevent these problems. Chemical methods of foam control are not always desired with respect to the problems (i.e., contamination, reduction of mass transfer) they may cause especially in food and pharmaceutical industries, where the product quality is of great importance. In order to prevent foam formation, in such cases mechanical methods are mostly dominant over chemical ones.
Speed of sound
The acoustical property of the speed of sound through a foam is of interest when analyzing failures of hydraulic components. The analysis involves calculating total hydraulic cycles to fatigue failure. The speed of sound in a foam is determined by the mechanical properties of the gas creating the foam: oxygen, nitrogen, or combinations.
An assumption that the speed of sound based on the fluid properties of the liquid will lead to errors in calculating fatigue cycles to failure of mechanical hydraulic components. Using acoustical transducers and related instrumentation that set low limits (0–50,000 Hz with roll-off) will result in errors. The low roll-off during measurement of actual frequency of acoustic cycles results in miscalculation due to actual hydraulic cycles in the possible ranges of 1–1000 MHz or higher. Instrumentation systems are most revealing when cycle bandwidths exceed the actual measured cycles by a factor of 10 to 100. Associated instrumentation costs also increase by factors of 10 to 100.
Most moving hydro-mechanical components cycle at 0–50 Hz, but entrained gas bubbles resulting in a foamy condition of the associated hydraulic fluid results in actual hydraulic cycles that can exceed 1000 MHz even if the moving mechanical components do not cycle at the higher cycle frequency.
Gallery
-

Close-up of sea foam (decomposing plankton) on a tide pool
-

Micrograph of temper (memory) foam
-

Diet Coke and Mentos foam "geyser"
-

Industrial CT scanning of a foam ball
-

Polystyrene foam cushioning
Foam scales and properties
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
References
- ↑ http://www.merriam-webster.com/dictionary/foam
- ↑ Lucassen, J. (1981). Lucassen-Reijnders, E. H., ed. Anionic Surfactants - Physical Chemistry of Surfactant Action. NY, USA: Marcel Dekker.
- ↑ Morgan, F. "Existence of Least-perimeter Partitions" (PDF).
- ↑ Bikerman,J,J, "Formation and Structure" in Foams New York, Springer-Verlag, 1973. ch 2. sec 24–25
- ↑ Jan. 2009. "IHC News- The Foam" [Online]. Available: http://www.clariant.com/C12575E4001FB2B8/vwLookupDownloads/2009_01_IHC_Newsletter_Defoaming.pdf/$FILE/2009_01_IHC_Newsletter_Defoaming.pdf
- ↑ Wilson, A.J, "Principles of Foam Formation and Stability." Foams: Physics, Chemistry, and Structure. New York, Springer-Verlag, 1989, ch 1
- ↑ Bikerman, J,J, "Formation and Structure" in Foams New York, Springer -Verlag, 1973. ch 2. sec 24–25
- ↑ I. Roland, G. Piel, L. Delattre, B. Evrard International Journal of Pharmaceutics 263 (2003) 85–94
- ↑ C. Lemarchand, P. Couvreur, M. Besnard, D. Costantini, R. Gref, Pharmaceutical Research, 20-8 (2003) 1284–1292
- ↑ O. Mengual, G. Meunier, I. Cayre, K. Puech, P. Snabre, Colloids and Surfaces A: Physicochemical and Engineering Aspects 152 (1999) 111–123
- ↑ P. Bru, L. Brunel, H. Buron, I. Cayré, X. Ducarre, A. Fraux, O. Mengual, G. Meunier, A. de Sainte Marie and P. Snabre Particle sizing and characterisation Ed T. Provder and J. Texter (2004)
- ↑ Shuo Wang, Peter Austin, Sumana Chakrabarti-Bell, 2003. It’s a maze: The pore structure of bread crumbs. Journal of Cereal Sciences, 54, 203-210
- ↑ Ashida, Kaneyoshi (2006). Polyurethane and related foams: chemistry and technology. CRC Press. pp. 79–81. ISBN 978-1-58716-159-9.
External links
| Look up foam in Wiktionary, the free dictionary. |
| Wikimedia Commons has media related to Foam. |
- Three-dimensional models for monodisperse foams: Cell aggregates and soap films (require Java)
- Aqueous foam technology
- The strange physics of foam
- Moriarty, Philip (2010). "Foam Physics". Sixty Symbols. Brady Haran for the University of Nottingham.















