Fluralaner
 | |
| Names | |
|---|---|
| IUPAC name
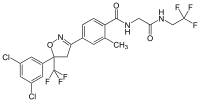
4-[(5R/S)-5-(3,5-Dichlorophenyl)-4,5-dihydro-5-trifluoromethyl-1,2-oxazol-3-yl]-N-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]-o-toluamide | |
| Identifiers | |
| 864731-61-3 (A) | |
| ChemSpider | 29398949 |
| Jmol interactive 3D | Image |
| |
| |
| Properties | |
| C22H17Cl2F6N3O3 | |
| Molar mass | 556.29 g·mol−1 |
| Pharmacology | |
| ATCvet code | QP53 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Fluralaner is a systemic insecticide and acaricide that is administered orally.[1] The US Food and Drug Administration (FDA) approved it under the trade name Bravecto for flea treatment in dogs in May 2014.[2] The EU approved the drug in February 2014.[3]
Mode of action
Fluralaner inhibits γ-aminobutyric acid (GABA)-gated chloride channels (GABACls) and l-glutamate-gated chloride channels (GluCls).[4]
References
- ↑ Walther, FM; Allan, MJ; Roepke, RK; Nuernberger, MC. "Safety of fluralaner chewable tablets (Bravecto), a novel systemic antiparasitic drug, in dogs after oral administration.". NCBI PubMed. National Center for Biotechnology Information. Retrieved 7 June 2015.
- ↑ Lee, Justin (21 May 2014). "New flea/tick medication by Merck just approved: Bravecto". Retrieved 3 Nov 2014.
- ↑ "MSD Animal Health receives EU approval for Bravecto". 19 Feb 2014. Retrieved 3 Nov 2014.
- ↑ Gassel1, Michael; Wolf, Christian; Noack, Sandra; Williams, Heike; Ilg, Thomas (February 2014). "The novel isoxazoline ectoparasiticide fluralaner: Selective inhibition of arthropod γ-aminobutyric acid- and l-glutamate-gated chloride channels and insecticidal/acaricidal activity". Insect Biochemistry and Molecular Biology 45: 111–124. doi:10.1016/j.ibmb.2013.11.009.
This article is issued from Wikipedia - version of the Thursday, January 07, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.