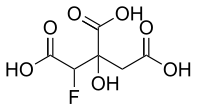
Fluorocitric acid
 | |
| Names | |
|---|---|
| IUPAC name
3-C-Carboxy-2,4-dideoxy-2-fluoropentaric acid | |
| Other names
2-Fluorocitric acid; 2-Fluorocitrate; 1-Fluoro-2-hydroxypropane-1,2,3-tricarboxylic acid | |
| Identifiers | |
| 357-89-1 | |
| ChemSpider | 96829 |
| Jmol interactive 3D | Image |
| PubChem | 107647 |
| |
| |
| Properties | |
| C6H7FO7 | |
| Molar mass | 210.11 g·mol−1 |
| Appearance | Odorless, white chrystals |
| Density | 1.37 |
| Melting point | 35.2°C |
| Boiling point | 165°C |
| Soluable | |
| Hazards | |
| Main hazards |   |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fluorocitric acid is a fluorinated carboxylic acid derived from citric acid by substitution of one hydrogen by a fluorine atom. The appropriate anion is called fluorocitrate. It is a metabolite of fluoroacetic acid and is very toxic because it is not processable using aconitase in the citrate cycle (where fluorocitrate takes place of citrate as the substrate). The enzyme is inhibited and the cycle stops working.[1]
See also
References
External links
- PubChem: Fluorocitrate
- Human Metabolome Database (HMDB): Fluorocitric acid
- The Chemical and Biochemical Properties of Fluorocitric Acid Pdf
This article is issued from Wikipedia - version of the Tuesday, December 22, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.