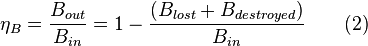Exergy efficiency
Exergy efficiency (also known as the second-law efficiency or rational efficiency) computes the efficiency of a process taking the second law of thermodynamics into account.
Motivation
From the second law of thermodynamics it can be demonstrated that no system can ever be 100% efficient. When calculating the energy efficiency of a system, the figure found gives no indication of how the system compares to a thermodynamically perfect one operating under the same conditions. In comparison, the rational efficiency of a system can reach 100% because the work output is compared to the potential of the input to do work. The energy efficiencies of a heat engine are always smaller than its exergy efficiency.
Definition
The exergy B balance of a process gives:
with exergy efficiency defined as:
For many engineering systems this can be rephrased as:
Where  is the standard Gibbs free energy of reaction at temperature
is the standard Gibbs free energy of reaction at temperature 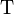 and pressure
and pressure  (also known as the standard Gibbs function change),
(also known as the standard Gibbs function change),  is the net work output and
is the net work output and 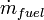 is the mass flow rate of fuel.
is the mass flow rate of fuel.
In the same way the energy efficiency can be defined as:
Where  is the standard enthalpy of reaction at temperature
is the standard enthalpy of reaction at temperature 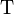 and pressure
and pressure  , for all fuels
, for all fuels  so the exergy efficiency must always be greater than the energy efficiency.
so the exergy efficiency must always be greater than the energy efficiency.
Application
The destruction of exergy is closely related to the creation of entropy and as such any system containing highly irreversible processes will have a low energy efficiency. As an example the combustion process inside a power stations gas turbine is highly irreversible and approximately 25% of the exergy input will be destroyed here.
For fossil fuels the free enthalpy of reaction is usually only slightly less than the enthalpy of reaction so from equations (3) and (4) we can see that the energy efficiency will be correspondingly larger than the energy law efficiency. For example, a typical combined cycle power plant burning methane may have an energy efficiency of 55%, while its exergy efficiency will be 57%. A 100% exergy efficient methane fired power station would correspond to an energy efficiency of 98%.
This means that for many of the fuels we use, the maximum efficiency that can be achieved is >90%, however we are restricted to the Carnot efficiency in many situations as a heat engine is being used.
Carnot
A common misconception is that the exergy efficiency compares a given cycle to a Carnot heat engine. This is incorrect because a Carnot engine is the most efficient heat engine possible, but not the most efficient device for creating work. Fuel cells, for instance, can theoretically reach much higher efficiencies than a Carnot engine.
Second law efficiency under maximum power
Neither first or second laws of thermodynamics include a measure of the rate of energy transformation. When a measure of the maximal rate of energy transformation is included in the measure of second law efficiency it is known as second law efficiency under maximum power, and directly related to the maximum power principle (Gilliland 1978, p. 101).
See also
References
- M.W. Gilliland (1978) Energy Analysis: A New Public Policy Tool, Westview Press.