Glyoxylic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
oxoethanoic acid | |
| Other names
formylformic acid; oxoethanoic acid | |
| Identifiers | |
| 298-12-4 | |
| ChEBI | CHEBI:16891 |
| ChEMBL | ChEMBL1162545 |
| ChemSpider | 740 |
| DrugBank | DB04343 |
| Jmol interactive 3D | Image |
| KEGG | C00048 |
| PubChem | 760 |
| |
| |
| Properties | |
| C2H2O3 | |
| Molar mass | 74.04 g·mol−1 |
| Density | 1.384 g/mL |
| Melting point | 80 °C (176 °F; 353 K)[1] |
| Boiling point | 111 °C (232 °F; 384 K) |
| Acidity (pKa) | 3.18,[2] 3.32 [3] |
| Related compounds | |
| Other anions |
glyoxylate |
| Related carboxylic acids |
formic acid acetic acid glycolic acid oxalic acid propionic acid pyruvic acid |
| Related compounds |
acetaldehyde glyoxal glycolaldehyde |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Structure and nomenclature
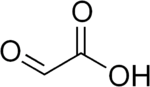
Glyoxylic acid is usually described with the chemical formula OCHCO2H, i.e. containing an aldehyde functional group (see image in upper right). In fact the aldehyde is not observed in solution or as a solid. In general aldehydes with electron-withdrawing substituents often exist mainly as their hydrate. Thus, the formula for glyoxylic acid is really (HO)2CHCO2H, described as the "monohydrate." This diol exists in equilibrium with the dimeric hemiacetal in solution:[4] Henry's law constant of glyoxylic acid is KH = 1.09 × 104 × exp[(40.0 × 103/R) × (1/T − 1/298)].[5]
- 2 (HO)2CHCO2H
 O[(HO)CHCO2H]2 + H2O
O[(HO)CHCO2H]2 + H2O
Occurrences and Preparations
The conjugate base of gloxylic acid is known as glyoxylate and is the form that the compound exists in solution at neutral pH. Glyoxylate is an intermediate of the glyoxylate cycle, which enables organisms, such as bacteria,[6] fungi, and plants [7] to convert fatty acids into carbohydrates. Glyoxylate is the byproduct of the amidation process in biosynthesis of several amidated peptides.
The compound is formed by organic oxidation of glyoxal with hot nitric acid, the main side product being oxalic acid.
Ozonolysis of maleic acid is also effective.[4]
Historically glyoxylic acid was prepared from oxalic acid electrosynthetically:[8][9]
In organic synthesis, lead dioxide anodes were applied for the production of glyoxylic acid from oxalic acid in a sulfuric acid electrolyte.[10]
Reactions and uses
Glyoxylic acid is about 10x stronger acid than acetic acid, with an acid dissociation constant of 4.7 × 10−4 (pKa = 3.32):
- (HO)2CHCOOH
 (HO)2CHCO2− + H+
(HO)2CHCO2− + H+
With base, glyoxylic acid disproportionates:
- 2 OCHCOOH + H2O → HOCH2COOH + HOOC–COOH
Even though the aldehyde is a very minor component of its solutions (as with formaldehyde solutions), glyoxylic acid behaves as an aldehyde in its reactions (again like formaldehyde). For example, it gives heterocycles upon condensation with urea and 1,2-diaminobenzene.
Phenol derivatives
Its condensation with phenols is versatile. The immediate product is 4-hydroxymandelic acid. This species reacts with ammonia to give hydroxyphenylglycine, a precursor to the drug amoxicillin. Reduction of the 4-hydroxymandelic acid gives 4-hydroxyphenylacetic acid, a precursor to the drug atenolol. Condensations with guaiacol in place of phenol provides a route to vanillin, a net formylation.[4][11][12]
Hopkins Cole reaction
Glyoxylic acid is one of the chemicals used in the Hopkins Cole reaction, used to check for the presence of tryptophan in proteins.
Safety
The compound is not very toxic with an LD50 for rats of 2500 mg/kg.
References
- ↑ Merck Index, 11th Edition, 4394
- ↑ Dissociation Constants Of Organic Acids and Bases (600 compounds), http://zirchrom.com/organic.htm.
- ↑ pKa Data Compiled by R. Williams, http://research.chem.psu.edu/brpgroup/pKa_compilation.pdf.
- 1 2 3 Georges Mattioda and Yani Christidis “Glyoxylic Acid” Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a12_495
- ↑ Ip, H. S. Simon; Huang, X. H. Hilda; Yu, Jian Zhen. "Effective Henry's law constants of glyoxal, glyoxylic acid, and glycolic acid". Geophysical Research Letters 36 (1). doi:10.1029/2008GL036212.
- ↑ Holms WH (1987). "Control of flux through the citric acid cycle and the glyoxylate bypass in Escherichia coli". Biochem Soc Symp. 54: 17–31. PMID 3332993.
- ↑ Escher CL, Widmer F (1997). "Lipid mobilization and gluconeogenesis in plants: do glyoxylate cycle enzyme activities constitute a real cycle? A hypothesis". Biol Chem. 378 (8): 803–813. PMID 9377475.
- ↑ Tafel, Julius; and Friedrichs, Gustav (1904). "Elektrolytische Reduction von Carbonsäuren und Carbonsäureestern in schwefelsaurer Lösung". Berichte der deutschen chemischen Gesellschaft 37 (3): 3187–3191. doi:10.1002/cber.190403703116. Retrieved 19 December 2013.
- ↑ Cohen, Julius (1920). Practical Organic Chemistry 2nd Ed. (PDF). London: Macmillan and Co. Limited. pp. 102–104.
- ↑ François Cardarelli (2008). Materials Handbook: A Concise Desktop Reference. Springer. p. 574. ISBN 1-84628-668-9.
- ↑ Fatiadi, Alexander; and Schaffer, Robert (1974). "An Improved Procedure for Synthesis of DL-4-Hydroxy-3-methoxymandelic Acid (DL-"Vanillyl"-mandelic Acid, VMA)" (PDF). Journal of Research of the National Bureau of Standards - A. Physics and Chemistry 78A (3): 411–412. doi:10.6028/jres.078A.024. Retrieved 19 December 2013. line feed character in
|title=at position 39 (help) - ↑ Kamlet, Jonas; and Mathieson, Olin (1953). Manufacture of vanillin and its homologues U.S. Patent 2,640,083 (PDF). U.S. Patent Office.
|
