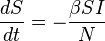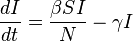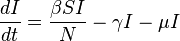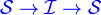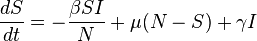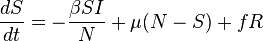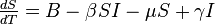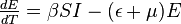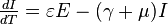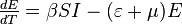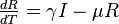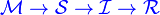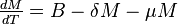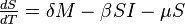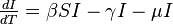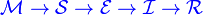Epidemic model
An epidemic model is a simplified means of describing the transmission of communicable disease through individuals.
Introduction
The modeling of infectious diseases is a tool which has been used to study the mechanisms by which diseases spread, to predict the future course of an outbreak and to evaluate strategies to control an epidemic (Daley & Gani, 2005).
The first scientist who systematically tried to quantify causes of death was John Graunt in his book Natural and Political Observations made upon the Bills of Mortality, in 1662. The bills he studied were listings of numbers and causes of deaths published weekly. Graunt’s analysis of causes of death is considered the beginning of the “theory of competing risks” which according to Daley and Gani (Daley & Gani, 2005, p. 2) is “a theory that is now well established among modern epidemiologists”.
The earliest account of mathematical modeling of spread of disease was carried out in 1766 by Daniel Bernoulli. Trained as a physician, Bernoulli created a mathematical model to defend the practice of inoculating against smallpox (Hethcote, 2000). The calculations from this model showed that universal inoculation against smallpox would increase the life expectancy from 26 years 7 months to 29 years 9 months (Bernoulli & Blower, 2004).
Daniel Bernoulli's work preceded our modern understanding of germ theory, and it was not until the research of Ronald Ross into the spread of malaria, that modern theoretical epidemiology began. This was soon followed by the work of A. G. McKendrick and W. O. Kermack, whose paper A Contribution to the Mathematical Theory of Epidemics was published in 1927. A simple deterministic (compartmental) model was formulated in this paper. The model was successful in predicting the behavior of outbreaks very similar to that observed in many recorded epidemics (Brauer & Castillo-Chavez, 2001).
Types of epidemic models
Stochastic
"Stochastic" means being or having a random variable. A stochastic model is a tool for estimating probability distributions of potential outcomes by allowing for random variation in one or more inputs over time. Stochastic models depend on the chance variations in risk of exposure, disease and other illness dynamics. They are used when these fluctuations are important, as in small populations (Trottier & Philippe, 2001).
Deterministic
When dealing with large populations, as in the case of tuberculosis, deterministic or compartmental mathematical models are used. In the deterministic model, individuals in the population are assigned to different subgroups or compartments, each representing a specific stage of the epidemic. Letters such as M, S, E, I, and R are often used to represent different stages.
The transition rates from one class to another are mathematically expressed as derivatives, hence the model is formulated using differential equations. While building such models, it must be assumed that the population size in a compartment is differentiable with respect to time and that the epidemic process is deterministic. In other words, the changes in population of a compartment can be calculated using only the history used to develop the model (Brauer & Castillo-Chavez, 2001).
Another approach is through discrete analysis on a lattice (such as a two-dimensional square grid), where the updating is done through asynchronous single-site updates (Kinetic Monte Carlo) or synchronous updating (Cellular Automata). The lattice approach enables inhomogeneities and clustering to be taken into account. Lattice systems are usually studied through computer simulation, and are discussed in the Wikipedia page Epidemic models on lattices.
Terminology
The following is a summary of the notation used in this and the next sections.
-
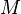 : Passively immune infants
: Passively immune infants -
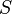 : Susceptibles
: Susceptibles -
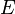 : Exposed individuals in the latent period
: Exposed individuals in the latent period -
 : Infectives
: Infectives -
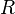 : Recovered with immunity
: Recovered with immunity -
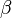 : Contact rate
: Contact rate -
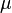 : Average death rate
: Average death rate -
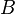 : Average birth rate
: Average birth rate -
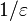 : Average latent period
: Average latent period -
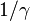 : Average infectious period
: Average infectious period -
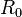 : Basic reproduction number
: Basic reproduction number -
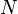 : Total population
: Total population -
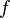 : Average loss of immunity rate of recovered individuals
: Average loss of immunity rate of recovered individuals -
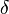 : Average temporary immunity period
: Average temporary immunity period
-
Deterministic compartmental models
The SIR model
In 1927, W. O. Kermack and A. G. McKendrick created a model in which they considered a fixed population with only three compartments: susceptible, 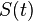 ; infected,
; infected, 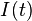 ; and removed,
; and removed,  . The compartments used for this model consist of three classes:[1]
. The compartments used for this model consist of three classes:[1]
-
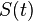 is used to represent the number of individuals not yet infected with the disease at time t, or those susceptible to the disease.
is used to represent the number of individuals not yet infected with the disease at time t, or those susceptible to the disease. -
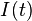 denotes the number of individuals who have been infected with the disease and are capable of spreading the disease to those in the susceptible category.
denotes the number of individuals who have been infected with the disease and are capable of spreading the disease to those in the susceptible category. -
 is the compartment used for those individuals who have been infected and then removed from the disease, either due to immunization or due to death. Those in this category are not able to be infected again or to transmit the infection to others.
is the compartment used for those individuals who have been infected and then removed from the disease, either due to immunization or due to death. Those in this category are not able to be infected again or to transmit the infection to others.
The flow of this model may be considered as follows:
Using a fixed population,  , Kermack and McKendrick derived the following equations:
, Kermack and McKendrick derived the following equations:
Several assumptions were made in the formulation of these equations: First, an individual in the population must be considered as having an equal probability as every other individual of contracting the disease with a rate of 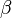 , which is considered the contact or infection rate of the disease. Therefore, an infected individual makes contact and is able to transmit the disease with
, which is considered the contact or infection rate of the disease. Therefore, an infected individual makes contact and is able to transmit the disease with 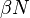 others per unit time and the fraction of contacts by an infected with a susceptible is
others per unit time and the fraction of contacts by an infected with a susceptible is 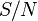 . The number of new infections in unit time per infective then is
. The number of new infections in unit time per infective then is 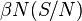 , giving the rate of new infections (or those leaving the susceptible category) as
, giving the rate of new infections (or those leaving the susceptible category) as 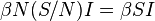 (Brauer & Castillo-Chavez, 2001). For the second and third equations, consider the population leaving the susceptible class as equal to the number entering the infected class. However, a number equal to the fraction (
(Brauer & Castillo-Chavez, 2001). For the second and third equations, consider the population leaving the susceptible class as equal to the number entering the infected class. However, a number equal to the fraction (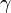 which represents the mean recovery/death rate, or
which represents the mean recovery/death rate, or 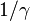 the mean infective period) of infectives are leaving this class per unit time to enter the removed class. These processes which occur simultaneously are referred to as the Law of Mass Action, a widely accepted idea that the rate of contact between two groups in a population is proportional to the size of each of the groups concerned (Daley & Gani, 2005). Finally, it is assumed that the rate of infection and recovery is much faster than the time scale of births and deaths and therefore, these factors are ignored in this model.
the mean infective period) of infectives are leaving this class per unit time to enter the removed class. These processes which occur simultaneously are referred to as the Law of Mass Action, a widely accepted idea that the rate of contact between two groups in a population is proportional to the size of each of the groups concerned (Daley & Gani, 2005). Finally, it is assumed that the rate of infection and recovery is much faster than the time scale of births and deaths and therefore, these factors are ignored in this model.
The SIR model with births and deaths
Using the case of for example, there is an arrival of new susceptible individuals into the population. For this type of situation births and deaths must be included in the model. The following differential equations represent this model, assuming a death rate 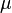 and birth rate equal to the death rate:
and birth rate equal to the death rate:
The SIS model with births and deaths
The SIS model can be easily derived from the SIR model by simply considering that the individuals recover with no immunity to the disease, that is, individuals are immediately susceptible once they have recovered.
Removing the equation representing the recovered population from the SIR model and adding those removed from the infected population into the susceptible population gives the following differential equations:
The SIRS model
This model is simply an extension of the SIR model as we will see from its construction.
The only difference is that it allows members of the recovered class to be free of infection and rejoin the susceptible class.
Models with more compartments
The SEIS model
The SEIS model takes into consideration the exposed or latent period of the disease, giving an additional compartment, E(t).
In this model an infection does not leave any immunity thus individuals that have recovered return to being susceptible again, moving back into the S(t) compartment. The following differential equations describe this model:
The SEIR model
The SIR model discussed above takes into account only those diseases which cause an individual to be able to infect others immediately upon their infection. Many diseases have what is termed a latent or exposed phase, during which the individual is said to be infected but not infectious.
In this model the host population (N) is broken into four compartments: susceptible, exposed, infectious, and recovered, with the numbers of individuals in a compartment, or their densities denoted respectively by S(t), E(t), I(t), R(t), that is N = S(t) + E(t) + I(t) + R(t)
The MSIR model
There are several diseases where an individual is born with a passive immunity from its mother.
To indicate this mathematically, an additional compartment is added, M(t), which results in the following differential equations:
The MSEIR model
For the case of a disease, with the factors of passive immunity, and a latency period there is the MSEIR model.
The MSEIRS model
An MSEIRS model is similar to the MSEIR, but the immunity in the R class would be temporary, so that individuals would regain their susceptibility when the temporary immunity ended.
Reproduction number
There is a threshold quantity which determines whether an epidemic occurs or the disease simply dies out. This quantity is called the basic reproduction number, denoted by R0, which can be defined as the number of secondary infections caused by a single infective introduced into a population made up entirely of susceptible individuals (S(0) ≈ N) over the course of the infection of this single infective. This infective individual makes β contacts per unit time producing new infections with a mean infectious period of 1/γ. Therefore, the basic reproduction number is
- R0 = β/γ
This value quantifies the transmission potential of a disease. If the basic reproduction number falls below one (R0 < 1), i.e. the infective may not pass the infection on during the infectious period, the infection dies out. If R0 > 1 there is an epidemic in the population. In cases where R0 = 1, the disease becomes endemic, meaning the disease remains in the population at a consistent rate, as one infected individual transmits the disease to one susceptible (Trottier & Philippe, 2001).
In cases of diseases with varying latent periods, the basic reproduction number can be calculated as the sum of the reproduction number for each transition time into the disease. An example of this is tuberculosis. Blower et al. (1995) calculated from a simple model of TB the following reproduction number:
- R0 = R0FAST + R0SLOW
In their model, it is assumed that the infected individuals can develop active TB by either direct progression (the disease develops immediately after infection) considered above as FAST tuberculosis or endogenous reactivation (the disease develops years after the infection) considered above as SLOW tuberculosis.
Other considerations within compartmental epidemic models
Vertical transmission
In the case of some diseases such as AIDS and Hepatitis B, it is possible for the offspring of infected parents to be born infected. This transmission of the disease down from the mother is called Vertical Transmission. The influx of additional members into the infected category can be considered within the model by including a fraction of the newborn members in the infected compartment (Brauer & Castillo-Chavez, 2001).
Vector transmission
Diseases transmitted from human to human indirectly, i.e. malaria spread by way of mosquitoes, are transmitted through a vector. In these cases, the infection transfers from human to insect and an epidemic model must include both species, generally requiring many more compartments than a model for direct transmission. For more information on this type of model see the reference Population Dynamics of Infectious Diseases: Theory and Applications, by R. M. Anderson (Brauer & Castillo-Chavez, 2001).
Others
Other occurrences (taken from Mathematical Models in Population Biology and Epidemiology by Fred Brauer and Carlos Castillo-Chávez ) which may need to be considered when modeling an epidemic include things such as the following:
- • Nonhomogeneous mixing
- • Age-structured populations
- • Variable infectivity
- • Distributions that are spatially non-uniform
- • Diseases caused by macroparasites
- • Acquired immunity through vaccinations
See also
- Mathematical modelling of infectious disease
- Compartmental models in epidemiology
- Epidemic
- Endemic (epidemiology)
- Next-generation matrix
- Transmission risks and rates
- Epidemic models on lattices
- Attack rate
References
- ↑ Kermack, W. O.; McKendrick, A. G. (1927). "A Contribution to the Mathematical Theory of Epidemics". Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 115 (772): 700. Bibcode:1927RSPSA.115..700K. doi:10.1098/rspa.1927.0118. JSTOR 94815.
- Anderson, R. M. ed. (1982) Population Dynamics of Infectious Diseases: Theory and Applications, Chapman and Hall, London-New York.
- Anderson, R. M. & May, R. M. (1991). Infectious Diseases of Humans. Oxford: Oxford University Press.
- Keeling, M.J. & Rohani, P. (2008). "Modeling Infectious Diseases: in Humans and Animals". Princeton, Princeton University Press.
- Bernoulli, D. & Blower, S. (2004). "An attempt at a new analysis of the mortality caused by smallpox and of the advantages of inoculation to prevent it." Reviews in Medical Virology, 14, 275 – 288.
- Blower, S. M., Mclean, A. R., Porco, T. C., Small, P. M., Hopewell, P. C., Sanchez, M. A., et al. (1995). "The intrinsic transmission dynamics of tuberculosis epidemics." Nature Medicine, 1, 815–821.
- Brauer, F. & Castillo-Chávez, C. (2001). Mathematical Models in Population Biology and Epidemiology. NY: Springer.
- Daley, D. J. & Gani, J. (2005). Epidemic Modeling: An Introduction. NY: Cambridge University Press.
- Hethcote, H. W. (2000). "The mathematics of infectious diseases." Society for Industrial and Applied Mathematics, 42, 599 – 653.
- Trottier, H., & Philippe, P. (2001). "Deterministic modeling of infectious diseases: theory and methods." The Internet Journal of Infectious Diseases.
Further reading
- An Introduction to Infectious Disease Modelling by Emilia Vynnycky and Richard G White. An introductory book on infectious disease modelling and its applications.
External links
Software
- AnyLogic
- Epigrass
- Model-Builder
- NetLogo
- STEM
- World Health Organization Public Health Mapping and GIS Program