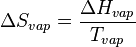Entropy of vaporization
The entropy of vaporization is the increase in entropy upon vaporization of a liquid. This is always positive since the degree of disorder increases in the transition from a liquid in a relatively small volume to a vapor or gas occupying a much larger space. At standard pressure Po = 1 bar, the value is denoted as ΔSovap and normally expressed in J mol−1 K−1.
In a phase transition such as vaporization, both phases coexist in equilibrium, so the difference in Gibbs free energy is equal to zero.
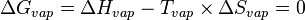 ,
,
where 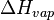 is the heat or enthalpy of vaporization. Since this is a thermodynamic equation, the symbol T refers to the absolute thermodynamic temperature, measured in Kelvin (K). The entropy of vaporization is then equal to the heat of vaporization divided by the boiling point.
is the heat or enthalpy of vaporization. Since this is a thermodynamic equation, the symbol T refers to the absolute thermodynamic temperature, measured in Kelvin (K). The entropy of vaporization is then equal to the heat of vaporization divided by the boiling point.
According to Trouton's rule, the entropy of vaporization (at standard pressure) of most liquids is about 85 to 88 J mol−1 K−1.