Embedded atom model
In computational chemistry and computational physics, the embedded atom model, embedded-atom method or EAM, is an approximation describing the energy between atoms, an interatomic potential. The energy is a function of a sum of functions of the separation between an atom and its neighbors. In the original model, by Murray Daw and Mike Baskes, the latter functions represent the electron density. EAM is related to the second moment approximation to tight binding theory, also known as the Finnis-Sinclair model. These models are particularly appropriate for metallic systems. Embedded-atom methods are widely used in molecular dynamics simulations.
Model simulation
In a simulation, the potential energy of an atom, 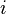 , is given by[1]
, is given by[1]
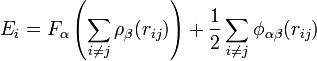 ,
,
where 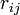 is the distance between atoms
is the distance between atoms 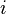 and
and 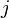 ,
, 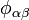 is a pair-wise potential function,
is a pair-wise potential function, 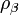 is the contribution to the electron charge density from atom
is the contribution to the electron charge density from atom 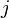 of type
of type 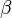 at the location of atom
at the location of atom 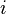 , and
, and 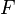 is an embedding function that represents the energy required to place atom
is an embedding function that represents the energy required to place atom 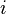 of type
of type 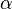 into the electron cloud.
into the electron cloud.
Since the electron cloud density is a summation over many atoms, usually limited by a cutoff radius, the EAM potential is a multibody potential. For a single element system of atoms, three scalar functions must be specified: the embedding function, a pair-wise interaction, and an electron cloud contribution function. For a binary alloy, the EAM potential requires seven functions: three pair-wise interactions (A-A, A-B, B-B), two embedding functions, and two electron cloud contribution functions. Generally these functions are provided in a tabularized format and interpolated by cubic splines.
See also
References
- ↑ "Pair - EAM". LAMMPS Molecular Dynamics Simulator. Retrieved 2008-10-01.
- Daw, Murray S.; Mike Baskes (1984). "Embedded-atom method: Derivation and application to impurities, surfaces, and other defects in metals". Physical Review B (American Physical Society) 29 (12): 6443–6453. Bibcode:1984PhRvB..29.6443D. doi:10.1103/PhysRevB.29.6443.
- Daw, Murray S.; Stephen Foiles (1993). "The embedded-atom method: a review of theory and applications". Mat. Sci. And Engr. Rep. 9 (7–8): 251. doi:10.1016/0920-2307(93)90001-U.