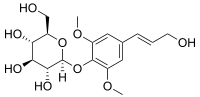
Syringin
 | |
| Names | |
|---|---|
| IUPAC name
4-[(1E)-3-Hydroxyprop-1-en-1-yl]-2,6-dimethoxyphenyl β-D-glucopyranoside | |
| Other names
Eleutheroside B; Ilexanthin A; Ligustrin; Lilacin; Magnolenin; Methoxyconiferine; Sinapyl alcohol 4-O-glucoside; Siringin; Syringoside | |
| Identifiers | |
| 118-34-3 | |
| ChEBI | CHEBI:9380 |
| ChemSpider | 4475831 |
| Jmol interactive 3D | Image |
| PubChem | 5316860 |
| |
| |
| Properties | |
| C17H24O9 | |
| Molar mass | 372.37 g·mol−1 |
| Appearance | White crystalline solid |
| Melting point | 192 °C (378 °F; 465 K)[1] |
| Slightly soluble[1] | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Syringin is a natural chemical compound first isolated from the bark of lilac (Syringa vulgaris) by Meillet in 1841.[1] It has since been found to be distributed widely throughout many types of plants. It is also called eleutheroside B, and is found in Eleutherococcus senticosus (Siberian ginseng). It is also found in dandelion coffee.
Chemically, it is the glucoside of sinapyl alcohol.
References
- 1 2 3 Merck Index, 11th Edition, 8997
This article is issued from Wikipedia - version of the Monday, November 02, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.