Electrospray ionization

Electrospray ionization (ESI) is a technique used in mass spectrometry to produce ions using an electrospray in which a high voltage is applied to a liquid to create an aerosol. It is especially useful in producing ions from macromolecules because it overcomes the propensity of these molecules to fragment when ionized. ESI is different from other atmospheric pressure ionization processes (e.g. MALDI) since it may produce multiply charged ions, effectively extending the mass range of the analyser to accommodate the kDa-MDa orders of magnitude observed in proteins and their associated polypeptide fragments.[1][2]
Mass spectrometry using ESI is called electrospray ionization mass spectrometry (ESI-MS) or, less commonly, electrospray mass spectrometry (ES-MS). ESI is a so-called 'soft ionization' technique, since there is very little fragmentation. This can be advantageous in the sense that the molecular ion (or more accurately a pseudo molecular ion) is always observed, however very little structural information can be gained from the simple mass spectrum obtained. This disadvantage can be overcome by coupling ESI with tandem mass spectrometry (ESI-MS/MS). Another important advantage of ESI is that solution-phase information can be retained into the gas-phase.
The electrospray ionization technique was first reported by Masamichi Yamashita and John Fenn in 1984.[3] The development of electrospray ionization for the analysis of biological macromolecules[4] was rewarded with the attribution of the Nobel Prize in Chemistry to John Bennett Fenn in 2002.[5] One of the original instruments used by Dr. Fenn is on display at the Chemical Heritage Foundation in Philadelphia, Pennsylvania.
History

In 1882, Lord Rayleigh theoretically estimated the maximum amount of charge a liquid droplet could carry before throwing out fine jets of liquid.[6] This is now known as the Rayleigh limit.
In 1914, John Zeleny published work on the behaviour of fluid droplets at the end of glass capillaries and presented evidence for different electrospray modes.[7] Wilson and Taylor [8] and Nolan investigated electrospray in the 1920s[9] and Macky in 1931.[10] The electrospray cone (now known as the Taylor cone) was described by Sir Geoffrey Ingram Taylor.[11]
The first use of electrospray ionization with mass spectrometry was reported by Malcolm Dole in 1968.[12][13] John Bennett Fenn was awarded the 2002 Nobel Prize in Chemistry for the development of electrospray ionization mass spectrometry in the late 1980s.[14]
Ionization mechanism

The liquid containing the analyte(s) of interest is dispersed by electrospray,[15] into a fine aerosol. Because the ion formation involves extensive solvent evaporation (also termed desolvation), the typical solvents for electrospray ionization are prepared by mixing water with volatile organic compounds (e.g. methanol [16] acetonitrile). To decrease the initial droplet size, compounds that increase the conductivity (e.g. acetic acid) are customarily added to the solution. These species also act to provide a source of protons to facilitate the ionization process. Large-flow electrosprays can benefit from nebulization a heated inert gas such as nitrogen or carbon dioxide in addition to the high temperature of the ESI source.[17] The aerosol is sampled into the first vacuum stage of a mass spectrometer through a capillary carrying a potential difference of approximately 3000V, which can be heated to aid further solvent evaporation from the charged droplets. The solvent evaporates from a charged droplet until it becomes unstable upon reaching its Rayleigh limit. At this point, the droplet deforms as the electrostatic repulsion of like charges, in an ever-decreasing droplet size, becomes more powerful than the surface tension holding the droplet together.[18] At this point the droplet undergoes Coulomb fission, whereby the original droplet 'explodes' creating many smaller, more stable droplets. The new droplets undergo desolvation and subsequently further Coulomb fissions. During the fission, the droplet loses a small percentage of its mass (1.0–2.3%) along with a relatively large percentage of its charge (10–18%).[19][20]
There are two major theories that explain the final production of gas-phase ions: the ion evaporation model (IEM) and the charge residue model (CRM). The IEM suggests that as the droplet reaches a certain radius the field strength at the surface of the droplet becomes large enough to assist the field desorption of solvated ions.[21][22] The CRM suggests that electrospray droplets undergo evaporation and fission cycles, eventually leading progeny droplets that contain on average one analyte ion or less.[12] The gas-phase ions form after the remaining solvent molecules evaporate, leaving the analyte with the charges that the droplet carried.
A large body of evidence, which is consider either direct or indirect that small ions (from small molecules) are liberated into the gas phase through the ion evaporation mechanism,[22][23] [24] while larger ions (from folded proteins for instance) form by charged residue mechanism [25][26][27]
A third model invoking combined charged residue-field emission has been proposed.[28] Another model called chain ejection model (CEM) is proposed for disordered polymers (unfolded proteins).[29]
The ions observed by mass spectrometry may be quasimolecular ions created by the addition of a hydrogen cation and denoted [M + H]+, or of another cation such as sodium ion, [M + Na]+, or the removal of a hydrogen nucleus, [M − H]−. Multiply charged ions such as [M + nH]n+ are often observed. For large macromolecules, there can be many charge states, resulting in a characteristic charge state envelope. All these are even-electron ion species: electrons (alone) are not added or removed, unlike in some other ionization sources. The analytes are sometimes involved in electrochemical processes, leading to shifts of the corresponding peaks in the mass spectrum. This effect is demonstrated in the direct ionization of noble metals such as copper, silver and gold using electrospray.[30]
Variants
The electrosprays operated at low flow rates generate much smaller initial droplets, which ensure improved ionization efficiency. In 1993 Gale and Richard D. Smith reported significant sensitivity increases could be achieved using lower flow rates, and down to 200 nL/min.[31] In 1994, two research groups coined the name micro-electrospray (microspray) for electrosprays working at low flow rates. Emmett and Caprioli demonstrated improved performance for HPLC-MS analyses when the electrospray was operated at 300–800 nL/min.[32] Wilm and Mann demonstrated that a capillary flow of ~ 25 nL/min can sustain an electrospray at the tip of emitters fabricated by pulling glass capillaries to a few micrometers.[33] The latter was renamed nano-electrospray (nanospray) in 1996.[34][35] Currently the name nanospray is also in use for electrosprays fed by pumps at low flow rates,[36] not only for self-fed electrosprays. Although there may not be a well-defined flow rate range for electrospray, microspray, and nano-electrospray,[37] studied "changes in analyte partition during droplet fission prior to ion release" .[37] In this paper, they compare results obtained by three other groups.[38][39][40] and then measure the signal intensity ratio [Ba2+ + Ba+]/[BaBr+] at different flow rates.
Cold spray ionization is a form of electrospray in which the solution containing the sample is forced through a small cold capillary (10-80 °C) into an electric field to create a fine mist of cold charged droplets.[41] Applications of this method include the analysis of fragile molecules and guest-host interactions that cannot be studied using regular electrospray ionization.
Electrospray ionization has also been achieved at pressures as low as 25 torr and termed subambient pressure ionization with nanoelectrospray (SPIN) based upon a two-stage ion funnel interface developed by Richard D. Smith and coworkers.[42] The SPIN implementation provided increased sensitivity due to the use of ion funnels that helped confine and transfer ions to the lower pressure region of the mass spectrometer. Operation at low pressure was particularly effective for low flow rates where the smaller electrospray droplet size allowed effective desolvation and ion formation to be achieved. As a result, later the researchers were later able to demonstrate achieving in excess of 50% overall ionization utilization efficiency for transfer of ions from the liquid phase, into the gas phase as ions, and through the dual ion funnel interface to the mass spectrometer.[43]
Ambient ionization
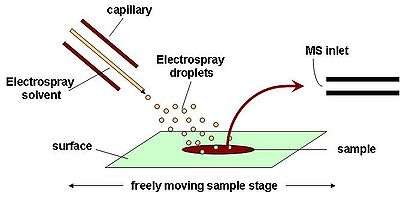
In ambient ionization, the formation of ions occurs outside the mass spectrometer without sample preparation.[44][45][46] Electrospray is used for ion formation is a number of ambient ion sources.
Desorption electrospray ionization (DESI) is an ambient ionization technique in which a solvent electrospray is directed at a sample.[47][48] The electrospray is attracted to the surface by applying a voltage to the sample. Sample compounds are extracted into the solvent which is again aerosolized as highly charged droplets that evaporate to form highly charged ions. After ionization, the ions enter the atmospheric pressure interface of the mass spectrometer. DESI allows for ambient ionization of samples at atmospheric pressure, with little sample preparation.
Extractive electrospray ionization is an spray-type, ambient ionization method that uses two merged sprays, one of which is generated by electrospray.[45]
Laser-based electrospray-based ambient ionization is a two-step process in which a pulsed laser is used to desorb or ablate material from a sample and the plume of material interacts with an electrospray to create ions.[45] For ambient ionization, the sample material is deposited on a target near the electrospray. The laser desorbs or ablates material from the sample which is ejected from the surface and into the electrospray which produces highly charged ions. Examples are electrospray laser desorption ionization, matrix-assisted laser desorption electrospray ionization, and laser ablation electrospray ionization
Applications

Electrospray is used to study protein folding.[49][50][51] Additionally, ESI-MS is used to test for the presence of nano clusters, for example U-60 (citation needed).
Liquid chromatography–mass spectrometry (LC-MS)
Electrospray ionization is the ion source of choice to couple liquid chromatography with mass spectrometry. The analysis can be performed online, by feeding the liquid eluting from the LC column directly to an electrospray, or offline, by collecting fractions to be later analyzed in a classical nanoelectrospray-mass spectrometry setup. Among the numerous operating parameters in ESI-MS,[52] the electrospray voltage has been identified as an important parameter to consider in ESI LC/MS gradient elution.[53] The effect of various solvent compositions [54] (such as TFA[55] or ammonium acetate,[20] or supercharging reagents,[56][57][58][59] or derivitizing groups[60] ) or spraying conditions[61] on Electrospray-LCMS spectra and/or nanoESI-MS spectra.[62] have been studied.
Capillary electrophoresis-mass spectrometry (CE-MS)
Capillary electrophoresis-mass spectrometry was enabled by an ESI interface that was developed and patented by Richard D. Smith and coworkers at Pacific Northwest National Laboratory, and shown to have broad utility for the analysis of very small biological and chemical compound mixtures, and even extending to a single biological cell.
Noncovalent gas phase interactions
Electrospray ionization is also utilized in studying noncovalent gas phase interactions. The electrospray process is thought to be capable of transferring liquid-phase noncovalent complexes into the gas phase without disrupting the noncovalent interaction. Problems[20][63] such as non specific interactions[64] have been identified when studying ligand substrate complexes by ESI-MS or nanoESI-MS. An interesting example of this is studying the interactions between enzymes and drugs which are inhibitors of the enzyme.[65][66][67] Competition studies between STAT6 and inhibitors[67][68][69] have used ESI as a way to screen for potential new drug candidates.
See also
References
- ↑ Ho, CS; Chan MHM; Cheung RCK; Law LK; Lit LCW; Ng KF; Suen MWM; Tai HL (February 2003). "Electrospray Ionisation Mass Spectrometry: Principles and Clinical Applications". Clin Biochem Rev 24 (1): 3–12. PMC 1853331. PMID 18568044.
- ↑ Pitt, James J (February 2009). "Principles and Applications of Liquid Chromatography-Mass Spectrometry in Clinical Biochemistry". Clin Biochem Rev 30 (1): 19–34. PMC 2643089. PMID 19224008.
- ↑ Yamashita, Masamichi; Fenn, John B. (September 1984). "Electrospray ion source. Another variation on the free-jet theme". The Journal of Physical Chemistry 88 (20): 4451–4459. doi:10.1021/j150664a002.
- ↑ Fenn, J. B.; Mann, M.; Meng, C. K.; Wong, S. F.; Whitehouse, C. M. (1989). "Electrospray ionization for mass spectrometry of large biomolecules". Science 246 (4926): 64–71. Bibcode:1989Sci...246...64F. doi:10.1126/science.2675315. PMID 2675315.
- ↑ Markides, K; Gräslund, A. "Advanced information on the Nobel Prize in Chemistry 2002" (PDF).
- ↑ Rayleigh, L. (1882). "On the Equilibrium of Liquid Conducting Masses charged with Electricity". Philosophical Magazine 14: 184–186. doi:10.1080/14786448208628425.
- ↑ Zeleny, J. (1914). "The electrical discharge from liquid points, and a hydrostatic method of measuring the electric intensity at their surfaces.". Physical Review 3 (2): 69. Bibcode:1914PhRv....3...69Z. doi:10.1103/PhysRev.3.69.
- ↑ Wilson, C. T.; G. I Taylor (1925). "The bursting of soap bubbles in a uniform electric field". Proc. Cambridge Philos. Soc. 22 (5): 728. Bibcode:1925PCPS...22..728W. doi:10.1017/S0305004100009609.
- ↑ Nolan, J. J. (1926). Proc. R. Ir. Acad. Sect. A 37: 28. Missing or empty
|title=(help) - ↑ Macky, W. A. (October 1, 1931). "Some Investigations on the Deformation and Breaking of Water Drops in Strong Electric Fields". Proceedings of the Royal Society A 133 (822): 565–587. Bibcode:1931RSPSA.133..565M. doi:10.1098/rspa.1931.0168.
- ↑ Sir Geoffrey Taylor (1964). "Disintegration of Water Droplets in an Electric Field". Proceedings of the Royal Society A 280 (1382): 383. Bibcode:1964RSPSA.280..383T. doi:10.1098/rspa.1964.0151. JSTOR 2415876.
- 1 2 Dole M, Mack LL, Hines RL, Mobley RC, Ferguson LD, Alice MB (1968). "Molecular Beams of Macroions". Journal of Chemical Physics 49 (5): 2240–2249. Bibcode:1968JChPh..49.2240D. doi:10.1063/1.1670391.
- ↑ Birendra N. Pramanik; A.K. Ganguly; Michael L. Gross (28 February 2002). Applied Electrospray Mass Spectrometry: Practical Spectroscopy Series. CRC Press. pp. 4–. ISBN 978-0-8247-4419-9.
- ↑ "Press Release: The Nobel Prize in Chemistry 2002". The Nobel Foundation. 2002-10-09. Retrieved 2011-04-02.
- ↑ Pozniak BP, Cole RB (2007). "Current Measurements within the Electrospray Emitter". JASMS 18 (4): 737–748. doi:10.1016/j.jasms.2006.11.012. PMID 17257852.
- ↑ Olumee; et al. (1998). "Droplet Dynamics Changes in Electrostatic Sprays of Methanol-Water Mixtures". J. Phys. Chem. A 102 (46): 9154–9160. doi:10.1021/jp982027z.
- ↑ Fernández De La Mora J (2007). "The Fluid Dynamics of Taylor Cones". Annual Review of Fluid Mechanics 39: 217. Bibcode:2007AnRFM..39..217F. doi:10.1146/annurev.fluid.39.050905.110159.
- ↑ Cole, Richard B (2010). Electrospray and MALDI Mass Spectrometry: Fundamentals, Instrumentation, Practicalities, and Biological Applications (2 ed.). Wiley. p. 4. ISBN 978-0471741077.
- ↑ Li KY, Tu H, Ray AK (April 2005). "Charge limits on droplets during evaporation". Langmuir 21 (9): 3786–94. doi:10.1021/la047973n. PMID 15835938.
- 1 2 3 Kebarle P, Verkerk UH (2009). "Electrospray: from ions in solution to ions in the gas phase, what we know now". Mass Spectrom Rev 28 (6): 898–917. doi:10.1002/mas.20247. PMID 19551695.
- ↑ Iribarne JV, Thomson BA (1976). "On the evaporation of small ions from charged droplets". Journal of Chemical Physics 64 (6): 2287–2294. Bibcode:1976JChPh..64.2287I. doi:10.1063/1.432536.
- 1 2 Nguyen S, Fenn JB (January 2007). "Gas-phase ions of solute species from charged droplets of solutions". Proc. Natl. Acad. Sci. USA 104 (4): 1111–7. Bibcode:2007PNAS..104.1111N. doi:10.1073/pnas.0609969104. PMC 1783130. PMID 17213314.
- ↑ Gamero-Castaño M (2000). "Direct measurement of ion evaporation kinetics from electrified liquid surfaces". J. Chem. Phys. 113 (2): 815. Bibcode:2000JChPh.113..815G. doi:10.1063/1.481857.
- ↑ de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta 406: 93–104. doi:10.1016/S0003-2670(99)00601-7.
An evaluation of the electric field on the drop surface at the point when it just ceases to be spherical (yet carries the total ion charge z) indicates that small PEG ions may be formed by ion evaporation. The break observed in the charge distribution may perhaps mean that the shift from the Dole to the ion evaporation mechanism arises at m�104, though this inference is highly hypothetical.
replacement character in|quote=at position 343 (help) - ↑ de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta 406: 93–104. doi:10.1016/S0003-2670(99)00601-7.
- ↑ de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta 406: 93–104. doi:10.1016/S0003-2670(99)00601-7.
For most published data examined, zmax is between 65% and 110% of zR, providing strong support in favor of Dole’s charged residue mechanism, at least for masses from 3.3 kD up to 1.4 MD. Other large but less compact ions from proteins and linear chains of polyethylene glycols (PEGs) have zmax values considerably larger than zR, apparently implying that they also formas charged residues, though from non-spherical drops held together by the polymer backbone.
- ↑ de la Mora Fernandez (2000). "Electrospray ionization of large multiply charged species proceeds via Dole's charged residue mechanism". Analytica Chimica Acta 406: 93–104. doi:10.1016/S0003-2670(99)00601-7.
The data do show a nearly discontinuous jump in the observed m/z for a mass somewhere between 20,000 and 50,000, and it is tempting to conclude that this is due to a corresponding transition where the ionization mechanism shifts from one type to the other. This would correspond to a critical value of z in the vicinity of 50, with a corresponding electric field of 2.6 V/nm. Of course, this is entirely hypothetical, and there is yet no compelling evidence of any kind indicating that an ion with as many as 30 charges can be formed by field evaporation.
- ↑ Hogan CJ, Carroll JA, Rohrs HW, Biswas P, Gross ML (January 2009). "Combined charged residue-field emission model of macromolecular electrospray ionization". Anal. Chem. 81 (1): 369–77. doi:10.1021/ac8016532. PMC 2613577. PMID 19117463.
- ↑ Unraveling the Mechanism of Electrospray Ionization. Lars Konermann, Elias Ahadi, Antony D. Rodriguez and Siavash Vahidi, Anal. Chem., 2013, 85 (1), pages 2–9, doi:10.1021/ac302789c
- ↑ Li, Anyin; Qingjie Luo; So-Jung Park; R. Graham Cooks (17 March 2014). "Synthesis and Catalytic Reactions of Nanoparticles formed by Electrospray Ionization of Coinage Metals". Angewandte Chemie International Edition 53 (12): 3147–3150. doi:10.1002/anie.201309193. Retrieved 11 April 2014.
- ↑ Gale DC, Smith RD (1993). "Small Volume and Low Flow Rate Electrospray Ionization Mass Spectrometry for Aqueous Samples". Rapid Commun. Mass Spectrom. 7: 1017–1021. doi:10.1002/rcm.1290071111.
- ↑ Emmett MR, Caprioli RM (1994). "Micro-electrospray mass spectrometry: ultra-high-sensitivity analysis of peptides and proteins". J. Am. Soc. Mass Spectrom. 5 (7): 605–613. doi:10.1016/1044-0305(94)85001-1.
- ↑ Wilm MS, Mann M (1994). "Electrospray and Taylor-Cone theory, Dole's beam of macromolecules at last?". Int. J. Mass Spectrom. Ion Proc. 136 (2–3): 167–180. Bibcode:1994IJMSI.136..167W. doi:10.1016/0168-1176(94)04024-9.
- ↑ Wilm M, Mann M (1996). "Analytical properties of the nanoelectrospray ion source". Anal. Chem. 68 (1): 1–8. doi:10.1021/ac9509519. PMID 8779426.
- ↑ Gibson; Mugo, Samuel M.; Oleschuk, Richard D.; et al. (2009). "Nanoelectrospray emitters: Trends and perspective". Mass Spectrometry Reviews 28 (6): 918–936. doi:10.1002/mas.20248. PMID 19479726.
- ↑ Page JS, Marginean I, Baker ES, Kelly RT, Tang K, Smith RD (December 2009). "Biases in ion transmission through an electrospray ionization-mass spectrometry capillary inlet". J. Am. Soc. Mass Spectrom. 20 (12): 2265–72. doi:10.1016/j.jasms.2009.08.018. PMC 2861838. PMID 19815425.
- 1 2 Schmidt A, Karas M, Dülcks T (May 2003). "Effect of different solution flow rates on analyte ion signals in nano-ESI MS, or: when does ESI turn into nano-ESI?". J. Am. Soc. Mass Spectrom. 14 (5): 492–500. doi:10.1016/S1044-0305(03)00128-4. PMID 12745218.
- ↑ Wilm M. S., Mann M. (1994). "Electrospray and Taylor-Cone Theory, Dole's Beam of Macromolecules at Last?". Int. J. Mass Spectrom. Ion Processes 136 (2–3): 167–180. Bibcode:1994IJMSI.136..167W. doi:10.1016/0168-1176(94)04024-9.
- ↑ Fernandez de la Mora J., Loscertales I. G. (2006). "The Current Emitted by Highly Conducting Taylor Cones". J. Fluid Mech. 260: 155–184. Bibcode:1994JFM...260..155D. doi:10.1017/S0022112094003472.
- ↑ Pfeifer RJ, Hendricks (1968). "Parametric Studies of Electrohydrodynamic Spraying". AIAA J. 6 (3): 496–502. Bibcode:1968AIAAJ...6..496H. doi:10.2514/3.4525.
- ↑ RSC Chemical Methods Ontology, Cold-spray ionisation mass spectrometry
- ↑ Page JS, Tang K, Kelly RT, Smith RD, (2008). "A subambient pressure ionization with nanoelectrospray (SPIN) source and interface for improved sensitivity in mass spectrometry". Analytical Chemistry 80: 1800–1805. doi:10.1021/ac702354b.
- ↑ I. Marginean, J. S. Page, A. V. Tolmachev, K. Tang and R. D. Smith (2010). "Achieving 50% Ionization Efficiency in Subambient Pressure Ionization with Nanoelectrospray". Analytical Chemistry 82: 9344–9349. doi:10.1021/ac1019123.
- ↑ Cooks, R. Graham; Ouyang, Zheng; Takats, Zoltan; Wiseman, Justin M. (2006). "Ambient Mass Spectrometry". Science 311 (5767): 1566–70. Bibcode:2006Sci...311.1566C. doi:10.1126/science.1119426. PMID 16543450.
- 1 2 3 Monge, María Eugenia; Harris, Glenn A.; Dwivedi, Prabha; Fernández, Facundo M. (2013). "Mass Spectrometry: Recent Advances in Direct Open Air Surface Sampling/Ionization". Chemical Reviews 113 (4): 2269–2308. doi:10.1021/cr300309q. ISSN 0009-2665. PMID 23301684.
- ↑ Huang, Min-Zong; Yuan, Cheng-Hui; Cheng, Sy-Chyi; Cho, Yi-Tzu; Shiea, Jentaie (2010). "Ambient Ionization Mass Spectrometry". Annual Review of Analytical Chemistry 3 (1): 43–65. Bibcode:2010ARAC....3...43H. doi:10.1146/annurev.anchem.111808.073702. ISSN 1936-1327.
- ↑ Z. Takáts, J.M. Wiseman, B. Gologan, R.G. Cooks (2004). "Mass Spectrometry Sampling Under Ambient Conditions with Desorption Electrospray Ionization". Science 306 (5695): 471–473. Bibcode:2004Sci...306..471T. doi:10.1126/science.1104404. PMID 15486296.
- ↑ Takáts Z, Wiseman JM, Cooks RG (2005). "Ambient mass spectrometry using desorption electrospray ionization (DESI): instrumentation, mechanisms and applications in forensics, chemistry, and biology". Journal of mass spectrometry : JMS 40 (10): 1261–75. doi:10.1002/jms.922. PMID 16237663.
- ↑ Konermann, L; Douglas, DJ (1998). "Equilibrium unfolding of proteins monitored by electrospray ionization mass spectrometry: Distinguishing two-state from multi-state transitions". Rapid Communications in Mass Spectrometry 12 (8): 435–442. doi:10.1002/(SICI)1097-0231(19980430)12:8<435::AID-RCM181>3.0.CO;2-F. PMID 9586231.
- ↑ Nemes; Goyal, Samita; Vertes, Akos; et al. (2008). "Conformational and Noncovalent Complexation Changes in Proteins during Electrospray Ionization". Analytical Chemistry 80 (2): 387–395. doi:10.1021/ac0714359. PMID 18081323.
- ↑ Sobott; Robinson (2004). "Characterising electrosprayed biomolecules using tandem-MS—the noncovalent GroEL chaperonin assembly". International Journal of Mass Spectrometry 236 (1–3): 25–32. Bibcode:2004IJMSp.236...25S. doi:10.1016/j.ijms.2004.05.010.
- ↑ for proteins: Vaidyanathan S., Kell D.B., Goodacre R. (2004). "Selective detection of proteins in mixtures using electrospray ionization mass spectrometry: influence of instrumental settings and implications for proteomics". Analytical Chemistry 76 (17): 5024–5032. doi:10.1021/ac049684. PMID 15373437.
- ↑ Marginean I, Kelly RT, Moore RJ, Prior DC, LaMarche BL, Tang K, Smith RD (April 2009). "Selection of the optimum electrospray voltage for gradient elution LC-MS measurements". J. Am. Soc. Mass Spectrom. 20 (4): 682–8. doi:10.1016/j.jasms.2008.12.004. PMC 2692488. PMID 19196520.
- ↑ Iavarone; Jurchen, John C.; Williams, Evan R.; et al. (2000). "Effects of solvent on the maximum charge state and charge state distribution of protein ions produced by electrospray ionization". JASMS 11 (11): 976–985. doi:10.1016/S1044-0305(00)00169-0.
- ↑ Garcia (2005). "The effect of the mobile phase additives on sensitivity in the analysis of peptides and proteins by high-performance liquid chromatography–electrospray mass spectrometry". Journal of Chromatography B 825 (2): 111–123. doi:10.1016/j.jchromb.2005.03.041.
- ↑ Teo CA, Donald WA (May 2014). "Solution additives for supercharging proteins beyond the theoretical maximum proton-transfer limit in electrospray ionization mass spectrometry". Anal. Chem. 86 (9): 4455–62. doi:10.1021/ac500304r. PMID 24712886.
- ↑ Lomeli SH, Peng IX, Yin S, Loo RR, Loo JA (January 2010). "New reagents for increasing ESI multiple charging of proteins and protein complexes". J. Am. Soc. Mass Spectrom. 21 (1): 127–31. doi:10.1016/j.jasms.2009.09.014. PMC 2821426. PMID 19854660.
- ↑ Lomeli SH, Yin S, Ogorzalek Loo RR, Loo JA (April 2009). "Increasing charge while preserving noncovalent protein complexes for ESI-MS". J. Am. Soc. Mass Spectrom. 20 (4): 593–6. doi:10.1016/j.jasms.2008.11.013. PMC 2789282. PMID 19101165.
- ↑ Yin S, Loo JA (March 2011). "Top-Down Mass Spectrometry of Supercharged Native Protein-Ligand Complexes". Int J Mass Spectrom 300 (2–3): 118–122. Bibcode:2011IJMSp.300..118Y. doi:10.1016/j.ijms.2010.06.032. PMC 3076692. PMID 21499519.
- ↑ Krusemark CJ, Frey BL, Belshaw PJ, Smith LM (September 2009). "Modifying the charge state distribution of proteins in electrospray ionization mass spectrometry by chemical derivatization". J. Am. Soc. Mass Spectrom. 20 (9): 1617–25. doi:10.1016/j.jasms.2009.04.017. PMC 2776692. PMID 19481956.
- ↑ Nemes P, Goyal S, Vertes A (January 2008). "Conformational and noncovalent complexation changes in proteins during electrospray ionization". Anal. Chem. 80 (2): 387–95. doi:10.1021/ac0714359. PMID 18081323.
- ↑ Ramanathan R, Zhong R, Blumenkrantz N, Chowdhury SK, Alton KB (October 2007). "Response normalized liquid chromatography nanospray ionization mass spectrometry". J. Am. Soc. Mass Spectrom. 18 (10): 1891–9. doi:10.1016/j.jasms.2007.07.022. PMID 17766144.
- ↑ Gabelica V, Vreuls C, Filée P, Duval V, Joris B, Pauw ED (2002). "Advantages and drawbacks of nanospray for studying noncovalent protein-DNA complexes by mass spectrometry". Rapid Commun. Mass Spectrom. 16 (18): 1723–8. doi:10.1002/rcm.776. PMID 12207359.
- ↑ Daubenfeld T, Bouin AP, van der Rest G (September 2006). "A deconvolution method for the separation of specific versus nonspecific interactions in noncovalent protein-ligand complexes analyzed by ESI-FT-ICR mass spectrometry". J. Am. Soc. Mass Spectrom. 17 (9): 1239–48. doi:10.1016/j.jasms.2006.05.005. PMID 16793278.
- ↑ Rosu F, De Pauw E, Gabelica V (July 2008). "Electrospray mass spectrometry to study drug-nucleic acids interactions". Biochimie 90 (7): 1074–87. doi:10.1016/j.biochi.2008.01.005. PMID 18261993.
- ↑ Wortmann A, Jecklin MC, Touboul D, Badertscher M, Zenobi R (May 2008). "Binding constant determination of high-affinity protein-ligand complexes by electrospray ionization mass spectrometry and ligand competition". J Mass Spectrom 43 (5): 600–8. doi:10.1002/jms.1355. PMID 18074334.
- 1 2 Jecklin MC, Touboul D, Bovet C, Wortmann A, Zenobi R (March 2008). "Which electrospray-based ionization method best reflects protein-ligand interactions found in solution? a comparison of ESI, nanoESI, and ESSI for the determination of dissociation constants with mass spectrometry". J. Am. Soc. Mass Spectrom. 19 (3): 332–43. doi:10.1016/j.jasms.2007.11.007. PMID 18083584.
- ↑ Touboul D, Maillard L, Grässlin A, Moumne R, Seitz M, Robinson J, Zenobi R (February 2009). "How to deal with weak interactions in noncovalent complexes analyzed by electrospray mass spectrometry: cyclopeptidic inhibitors of the nuclear receptor coactivator 1-STAT6". J. Am. Soc. Mass Spectrom. 20 (2): 303–11. doi:10.1016/j.jasms.2008.10.008. PMID 18996720.
- ↑ Czuczy N, Katona M, Takats Z (February 2009). "Selective detection of specific protein-ligand complexes by electrosonic spray-precursor ion scan tandem mass spectrometry". J. Am. Soc. Mass Spectrom. 20 (2): 227–37. doi:10.1016/j.jasms.2008.09.010. PMID 18976932.
Further reading
- Cole, Richard (1997). Electrospray ionization mass spectrometry: fundamentals, instrumentation, and applications. New York: Wiley. ISBN 0-471-14564-5.
- Gross, Michael; Pramanik, Birendra N.; Ganguly, A. K. (2002). Applied electrospray mass spectrometry. New York, N.Y: Marcel Dekker. ISBN 0-8247-0618-8.
- Snyder, A. Peter (1996). Biochemical and biotechnological applications of electrospray ionization mass spectrometry. Columbus, OH: American Chemical Society. ISBN 0-8412-3378-0.
- Alexandrov, M. L.; L. N. Gall; N. V. Krasnov; V. I. Nikolaev; V. A. Pavlenko; V. A. Shkurov (July 1984). Экстракция ионов из растворов при атмосферном давлении – Метод масс-спектрометрического анализа биоорганических веществ [Extraction of ions from solutions at atmospheric pressure - A method for mass spectrometric analysis of bioorganic substances]. Doklady Akademii Nauk SSSR (in Russian) 277 (2): 379–383.
- Alexandrov, M. L.; L. N. Gall; N. V. Krasnov; V. I. Nikolaev; V. A. Pavlenko; V. A. Shkurov (2008) [July 1984]. "Extraction of ions from solutions under atmospheric pressure as a method for mass spectrometric analysis of bioorganic compounds". Rapid Communications in Mass Spectrometry 22 (3): 267–270. doi:10.1002/rcm.3113.
External links
- Electrospray Ionization Primer National High Magnetic Field Laboratory
- Electrospray Ionization Mass Spectrometry at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||||||||||