Electrometer
An electrometer is an electrical instrument for measuring electric charge or electrical potential difference. There are many different types, ranging from historical handmade mechanical instruments to high-precision electronic devices. Modern electrometers based on vacuum tube or solid-state technology can be used to make voltage and charge measurements with very low leakage currents, down to 1 femtoampere. A simpler but related instrument, the electroscope, works on similar principles but only indicates the relative magnitudes of voltages or charges.
Older electrometers
Gold-leaf electrometer

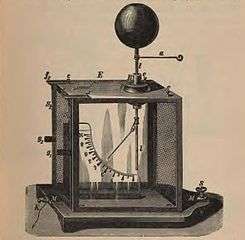
The gold-leaf electroscope was one of the first sensitive instruments used to indicate electric charge. It is still used for science demonstrations but has been superseded in most applications by electronic measuring instruments. The instrument consists of two thin leaves of gold foil suspended from an electrode. When the electrode is charged by induction or by contact, the leaves acquire similar electric charges and repel each other due to the Coulomb force. Their separation is a direct indication of the net charge stored on them. On the glass opposite the leaves, pieces of tin foil may be pasted, so that when the leaves diverge fully they may discharge into the ground. The leaves may be enclosed in a glass envelope to protect them from drafts, and the envelope may be evacuated to minimize charge leakage. A further cause of charge leakage is ionizing radiation, so to prevent this, the electrometer must be surrounded by lead shielding. This principle has been used to detect ionizing radiation, as seen in the quartz fibre electrometer and Kearny fallout meter.
This type of electroscope usually acts as an indicator and not a measuring device, although it can be calibrated. The Braun electroscope replaced the gold-leaf electroscope for more accurate measurements.
The instrument was developed in the 18th century by several researchers, among them Abraham Bennet and Alessandro Volta.
Early quadrant electrometer

While the term "quadrant electrometer" eventually referred to Kelvin's version, this term was first used to describe a simpler device.[1] It consists of an upright stem of wood, to which is affixed to a semicircle of ivory. From the center there hangs a light cork ball upon a pivot. When the instrument is placed upon a charged body, the stem participates and repels the cork ball. The amount of repulsion may be read off the graduated semicircle, though it is obvious that the measured angle is not in direct proportion to the charge.
Coulomb's electrometer

This design uses torsion to give a measurement more sensitive than repulsion of gold leaves or cork-balls. It consists of a glass cylinder with a glass tube on top. In the axes of the tube is a glass thread, the lower end of this holds a bar of gum lac, with a gilt pith ball at each extremity. Through another aperture on the cylinder, another gum lac rod with gilt balls may be introduced. This is called the carrier rod.
If the lower ball of the carrier rod is charged when it is entered into the aperture, this will repel one of the movable balls inside. An index and scale (not pictured) is attached to the top of the twistable glass rod. The number of degrees twisted to bring the balls back together is in exact proportion of the amount of charge of the ball of the carrier rod.
Peltier electrometer
Developed by Peltier, this uses a form of magnetic compass to measure deflection by balancing the electrostatic force with a magnetic needle.
Bohnenberger electrometer
The Bohnenberger electrometer, developed by J.G.F. von Bohnenberger, consists of a single gold leaf suspended vertically between the anode and cathode of a dry pile. Any charge imparted to the gold leaf causes it to move toward one or the other pole; thus, the sign of the charge as well as its approximate magnitude may be gauged.[2]
Attraction electrometer
Also known as Attracted Disk Electrometers, attraction electrometers are sensitive balances measuring the attraction between charged disks. William Snow Harris is credited with the invention of this instrument, which was further improved by Lord Kelvin.
Kelvin's quadrant electrometer

Developed by Lord Kelvin, this is the most sensitive and accurate of all the mechanical electrometers. The original design uses a light aluminum sector suspended inside a drum cut into four segments. The segments are insulated and connected diagonally in pairs. The charged aluminum sector is attracted to one pair of segments and repelled from the other. The deflection is observed by a beam of light reflected from a small mirror attached to the sector, just as in a galvanometer. The engraving on the right shows a slightly different form of this electrometer, using four flat plates rather than closed segments. The plates can be connected externally in the conventional diagonal way (as shown), or in a different order for specific applications.
A more sensitive form of quadrant electrometer was developed by Frederick Lindemann. It employs a metal-coated quartz fiber instead of an aluminum sector. The deflection is measured by observing the movement of the fiber under a microscope. Initially used for measuring star light, it was employed for the infrared detection of airplanes in the early stages of World War II.
Some mechanic electrometers were housed inside a cage often referred to as a “bird cage”. This is a form of Faraday Cage that protected the instrument from external electrostatic charges.
Modern electrometers
A modern electrometer is a highly sensitive electronic voltmeter whose input impedance is so high that the current flowing into it can be considered, for most practical purposes, to be zero. The actual value of input resistance for modern electronic electrometers is around 1014Ω, compared to around 1010Ω for nanovoltmeters.[3][4] Due to the extremely high input impedance, special design considerations must be applied to avoid leakage current such as driven shields and special insulation materials.
Among other applications, electrometers are used in nuclear physics experiments as they are able to measure the tiny charges left in matter by the passage of ionizing radiation. The most common use for modern electrometers is the measurement of radiation with ionization chambers, in instruments such as geiger counters.
Vibrating reed electrometers
Vibrating reed electrometers use a variable capacitor formed between a moving electrode (in the form of a vibrating reed) and a fixed input electrode. As the distance between the two electrodes varies, the capacitance also varies and electric charge is forced in and out of the capacitor. The alternating current signal produced by the flow of this charge is amplified and used as an analogue for the DC voltage applied to the capacitor. The DC input resistance of the electrometer is determined solely by the leakage resistance of the capacitor, and is typically extremely high, (although its AC input impedance is lower).
For convenience of use, the vibrating reed assembly is often attached by a cable to the rest of the electrometer. This allows for a relatively small unit to be located near the charge to be measured while the much larger reed-driver and amplifier unit can be located wherever it is convenient for the operator.
Valve electrometers
Valve electrometers use a specialized vacuum tube (thermionic valve) with a very high gain (transconductance) and input resistance. The input current is allowed to flow into the high impedance grid, and the voltage so generated is vastly amplified in the anode (plate) circuit. Valves designed for electrometer use have leakage currents as low as a few femtoamperes (10−15 amperes). Such valves must be handled with gloved hands as the salts left on the glass envelope can provide leakage paths for these tiny currents.
In a specialized circuit called inverted triode, the roles of anode and grid are reversed. This places the control electrode at a maximum distance from the space-charge region surrounding the filament, minimizing the amount of electrons collected by the control electrode, and thus minimizing the input current.[5]
Solid-state electrometers
The most modern electrometers consist of a solid state amplifier using one or more field-effect transistors, connections for external measurement devices, and usually a display and/or data-logging connections. The amplifier amplifies small currents so that they are more easily measured. The external connections are usually of a co-axial or tri-axial design, and allow attachment of diodes or ionization chambers for ionising radiation measurement. The display or data-logging connections allow the user to see the data or record it for later analysis. Electrometers designed for use with ionization chambers may include a high-voltage power supply, which is used to power the ionization chamber.
Solid-state electrometers are often multipurpose devices that can measure voltage, charge, resistance and current. They measure voltage by means of "voltage balancing", in which the input voltage is compared with an internal reference voltage source using an electronic circuit with a very high input impedance (of the order of 1014 ohms). A similar circuit modified to act as a current-to-voltage converter enables the instrument to measure currents as small as a few femtoamperes. Combined with an internal voltage source, the current measuring mode can be adapted to measure very high resistances, of the order of 1017 ohms. Finally, by calculation from the known capacitance of the electrometer's input terminal, the instrument can measure very small electric charges, down to a small fraction of a picocoulomb.
See also
References
- Dr. J. Frick, Physical Technics; Or Practical Instructions for Making Experiments in Physics Translated By John D. Easter, Ph.D. - J. B. Lippincott & Co., Philadelphia 1862
- Robert Mfurgeson Ph.D. Electricity - William and Robert Chambers, London and Edinburgh 1866
- Silvanus P. Thompson, Elementary Lessons in electricity and Magnetism. - Macmillan and Co. Limited, London 1905
- Jones, R. V., Instruments and Experiences - John Wiley and Sons, London 1988
- ↑ Draper, John William (1861). A textbook on chemistry. New York: Harper and sons. p. 119.
- ↑ John Angell (1877). Elements of magnetism and electricity: with practical instructions for the performance of experiments, and the construction of cheap apparatus. W. Collins, Sons, & Co. pp. 169–. Retrieved 26 May 2013.
- ↑ Keithley, Making precision low current and high resistance measurements, "A greater measure of confidence" brochure, 2011, page 8
- ↑ Keithley, Nanovoltmeter 2182A, Datasheet, page 5
- ↑ Inverted Triode for Industrial Measurements, Manual of Industrial Circuits Electronics. Electronics. December 1944. p. 176.
External links
| Wikimedia Commons has media related to Electroscopes. |
- Build this FET electrometer - A very simple circuit - 2 components
- Simple FET electrometer - A simple bridged circuit
- An op-amp electrometer
- Early electrometers
- Charging an Electroscope by Induction Using a Negatively-Charged Balloon from Multimedia Physics Studios