Glycine
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Glycine | |||
| Other names
Aminoethanoic acid Aminoacetic acid Glycocoll | |||
| Identifiers | |||
| 56-40-6 | |||
| Abbreviations | Gly, G | ||
| ChEBI | CHEBI:15428 | ||
| ChEMBL | ChEMBL773 | ||
| ChemSpider | 730 | ||
| DrugBank | DB00145 | ||
| EC Number | 200-272-2 | ||
| 727 | |||
| Jmol interactive 3D | Image | ||
| KEGG | D00011 | ||
| PubChem | 750 | ||
| UNII | TE7660XO1C | ||
| |||
| |||
| Properties | |||
| C2H5NO2 | |||
| Molar mass | 75.07 g/mol | ||
| Appearance | white solid | ||
| Density | 1.607 g/cm3 | ||
| Melting point | 233 °C (451 °F; 506 K) (decomposition) | ||
| 24.99 g/100 mL (25 °C)[2] | |||
| Solubility | soluble in pyridine sparingly soluble in ethanol insoluble in ether | ||
| Acidity (pKa) | 2.34 (carboxyl), 9.6 (amino)[3] | ||
| Pharmacology | |||
| ATC code | B05 | ||
| Hazards | |||
| Safety data sheet | See: data page | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (Median dose) |
2600 mg/kg (mouse, oral) | ||
| Supplementary data page | |||
| Refractive index (n), Dielectric constant (εr), etc. | |||
| Thermodynamic data |
Phase behaviour solid–liquid–gas | ||
| UV, IR, NMR, MS | |||
| | |||
| Infobox references | |||
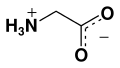
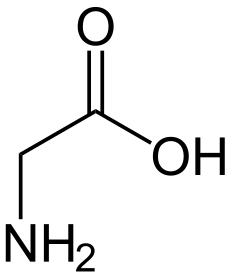
Glycine (abbreviated as Gly or G) is the smallest of the 20 amino acids commonly found in proteins, and indeed is the smallest possible (having a hydrogen substituent as its side-chain). The formula is NH2CH2COOH. Its codons are GGU, GGC, GGA, GGG of the genetic code.
Glycine is a colourless, sweet-tasting crystalline solid. It is unique among the proteinogenic amino acids in that it is achiral. It can fit into hydrophilic or hydrophobic environments , due to its minimal side chain of only one hydrogen atom.
History and etymology
Glycine was first isolated from gelatin in 1820.[4] The name comes from the ancient Greek word γλυκύς "sweet tasting".[5]
Production
Glycine was discovered in 1820, by Henri Braconnot who boiled a gelatinous object with sulfuric acid.[6]
Glycine is manufactured industrially by treating chloroacetic acid with ammonia:[7]
- ClCH2COOH + 2 NH3 → H2NCH2COOH + NH4Cl
About 15 million kg are produced annually in this way.[8]
In the USA (by GEO Specialty Chemicals, Inc.) and in Japan (by Showa Denko KK), glycine is produced via the Strecker amino acid synthesis.[9][10]
There are two producers of glycine in the United States: Chattem Chemicals, Inc., a subsidiary of Mumbai-based Sun Pharmaceutical, and GEO Specialty Chemicals, Inc., which purchased the glycine and naphthalene sulfonate production facilities of Hampshire Chemical Corp, a subsidiary of Dow Chemical.[9][11]
Chattem's manufacturing process ("MCA" process) occurs in batches and results in a finished product with some residual chloride but no sulfate, while GEO’s manufacturing process is considered a semi-batch process and results in a finished product with some residual sulfate but no chloride.
Glycine is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct.[12]
Acid-base properties and structures

In aqueous solution, glycine itself is amphoteric: at low pH the molecule can be protonated with a pKa of about 2.4 and at high pH it loses a proton with a pKa of about 9.6 (precise values of pKa depend on temperature and ionic strength). The nature of glycine in aqueous solution has been investigated by theoretical methods.[13] In solution the ratio of concentrations of the two isomers is independent of both the analytical concentration and of pH. This ratio is simply the equilibrium constant for isomerization.
Both isomers of glycine have been observed by microwave spectroscopy in the gas phase.[14] The solid-state structure has been analyzed in detail.[15]
Metabolism
Biosynthesis
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phosphoglycerate, but the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis.[16] In most organisms, the enzyme serine hydroxymethyltransferase catalyses this transformation via the cofactor pyridoxal phosphate:[17]
- serine + tetrahydrofolate → glycine + N5,N10-Methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible:[17]
- CO2 + NH4+ + N5,N10-Methylene tetrahydrofolate + NADH + H+ → Glycine + tetrahydrofolate + NAD+
Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35% glycine.[17][18]
Degradation
Glycine is degraded via three pathways. The predominant pathway in animals and plants involves the glycine cleavage enzyme:[17]
- Glycine + tetrahydrofolate + NAD+ → CO2 + NH4+ + N5,N10-Methylene tetrahydrofolate + NADH + H+
In the second pathway, glycine is degraded in two steps. The first step is the reverse of glycine biosynthesis from serine with serine hydroxymethyl transferase. Serine is then converted to pyruvate by serine dehydratase.[17]
In the third pathway of glycine degradation, glycine is converted to glyoxylate by D-amino acid oxidase. Glyoxylate is then oxidized by hepatic lactate dehydrogenase to oxalate in an NAD+-dependent reaction.[17]
The half-life of glycine and its elimination from the body varies significantly based on dose. In one study, the half-life was between 0.5 and 4.0 hours. [19]
Physiological function
The principal function of glycine is as a precursor to proteins, such as its periodically repeated role in the formation of the collagen helix in conjunction with Hydroxyproline. It is also a building block to numerous natural products.
As a biosynthetic intermediate
In higher eukaryotes, δ-aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA by the enzyme ALA synthase. Glycine provides the central C2N subunit of all purines.[17]
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required co-agonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutamatergic receptors which are excitatory.[20] The LD50 of glycine is 7930 mg/kg in rats (oral),[21] and it usually causes death by hyperexcitability.
A 2014 review on sleep aids noted that glycine can improve sleep quality, citing a study in which 3 grams of glycine before bedtime improved sleep quality in humans.[22][23] Glycine has also been positively tested as an add-on treatment for schizophrenia.[24]
Uses
In the US, glycine is typically sold in two grades: United States Pharmacopeia (“USP”), and technical grade. Most glycine is manufactured as USP grade material for diverse uses. USP grade sales account for approximately 80 to 85 percent of the U.S. market for glycine.
- Pharmaceutical grade glycine is produced for some pharmaceutical applications, such as intravenous injections, where the customer’s purity requirements often exceed the minimum required under the USP grade designation. Pharmaceutical grade glycine is often produced to proprietary specifications and is typically sold at a premium over USP grade glycine.
- Technical grade glycine, which may or may not meet USP grade standards, is sold for use in industrial applications; e.g., as an agent in metal complexing and finishing. Technical grade glycine is typically sold at a discount to USP grade glycine.[25]
Animal and human foods
Other markets for USP grade glycine include its use an additive in pet food and animal feed. For humans, glycine is sold as a sweetener/taste enhancer. Certain food supplements and protein drinks contain glycine.[26] Certain drug formulations include glycine to improve gastric absorption of the drug.[26]
Cosmetics and miscellaneous applications
Glycine serves as a buffering agent in antacids, analgesics, antiperspirants, cosmetics, and toiletries.
Many miscellaneous products use glycine or its derivatives, such as the production of rubber sponge products, fertilizers, metal complexants.[27]
Chemical feedstock
Glycine is an intermediate in the synthesis of a variety of chemical products. It is used in the manufacture of the herbicide glyphosate.
Research and Development
Glycine is a significant component of some solutions used in the SDS-PAGE method of protein analysis. It serves as a buffering agent, maintaining pH and preventing sample damage during electrophoresis. Glycine is also used to remove protein-labelling antibodies from Western Blot membranes to enable the probing of numerous proteins of interest from SDS-PAGE gel. This allows more data to be drawn from the same specimen, increasing the reliability of the data, reducing the amount of sample processing, and number of samples required. This process is known as 'stripping'.
Anti-aging properties
Glycine treatment may help reverse the age-associated defects in human fibroblast.[28] Two genes that regulate mitochondria, GCAT and SHMT2, were found to affect age-associated mitochondrial defects. By changing the regulation of these genes, researchers were able to restore mitochondrial function or induce more defects in the fibroblast cell lines. In an interesting finding,[29] the addition of glycine for 10 days to the culture medium of the 97-year-old fibroblast cell line restored its respiratory function.
Presence in space
The detection of glycine in the interstellar medium has been debated.[30] In 2008, the glycine-like molecule aminoacetonitrile was discovered in the Large Molecule Heimat, a giant gas cloud near the galactic center in the constellation Sagittarius by the Max Planck Institute for Radio Astronomy.[31] In 2009, glycine sampled in 2004 from comet Wild 2 by the NASA spacecraft Stardust was confirmed. This is the first discovery of glycine outside the Earth, however, glycine was identified in the Murchison meteorite in 1970.[32] That mission's results bolstered the theory of panspermia, which claims that the "building-blocks" of life are widespread throughout the universe.[33]
See also
References
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.), Merck, 1989, ISBN 091191028X, 4386.
- ↑ "Solubilities and densities". Prowl.rockefeller.edu. Retrieved 2013-11-13.
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ↑ "glycine". Encyclopaedia Britannica Online. Retrieved 2015-12-06.
- ↑ "glycine". Oxford Dictionaries. Retrieved 2015-12-06.
- ↑ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer & F.G. Hopkins, ed. The chemical composition of the proteins. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 82. Retrieved January 18, 2010.
- ↑ Ingersoll, A. W.; Babcock, S. H. (1932). "Hippuric acid". Org. Synth. 12: 40.; Coll. Vol. 2, p. 328
- ↑ Karlheinz Drauz, Ian Grayson, Axel Kleemann, Hans-Peter Krimmer, Wolfgang Leuchtenberger, Christoph Weckbecker "Amino Acids" in Ullmann's Encyclopedia of Industrial Chemistry 2007, Wiley-VCH, Weinheim. doi:10.1002/14356007.a02_057.pub2
- 1 2 "Glycine Conference (prelim)". USITC. Retrieved 2014-06-13.
- ↑ Archived June 13, 2013 at the Wayback Machine
- ↑ U.S. International Trade Commission, "Glycine From China." Investigation No. 731-TA-718 (Second Review), Publication No. 3810, October 2005
- ↑ Hart, J. Roger (2005) "Ethylenediaminetetraacetic Acid and Related Chelating Agents" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim. doi:10.1002/14356007.a10_095
- ↑ Bonaccorsi, R.; Palla, P.; Tomasi, J. (1984). "Conformational energy of glycine in aqueous solutions and relative stability of the zwitterionic and neutral forms. An ab initio study". J. Amer. Chem. Soc 106 (7): 1945–1950. doi:10.1021/ja00319a008.
- ↑ Suenram, R.D.; Lovas, F.J (1980). "Millimeter wave spectrum of glycine. A new conformer". J. Amer. Chem. Soc 102 (24): 7180–7184. doi:10.1021/ja00544a002.
- ↑ Jönsson, P.-G.; Kvick, Å (1972). "Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid -glycine". Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid -glycine B28 (6): 1827–1833. doi:10.1107/S0567740872005096.
- ↑ "A weak link in metabolism: the metabolic capacity for glycine biosynthesis does not satisfy the need for collagen synthesis.". Journal of biosciences. Dec 2009. PMID 20093739.
- 1 2 3 4 5 6 7 Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, pp. 127, 675–77, 844, 854, ISBN 0-7167-4339-6
- ↑ Szpak, Paul (2011). "Fish bone chemistry and ultrastructure: implications for taphonomy and stable isotope analysis". Journal of Archaeological Science 38 (12): 3358–3372. doi:10.1016/j.jas.2011.07.022.
- ↑ Hahn RG (1993). "Dose-dependent half-life of glycine". Urological Research 21 (4): 289–291. doi:10.1007/BF00307714. PMID 8212419.
- ↑ "Recent development in NMDA receptors". Chinese Medical Journal. 2000.
- ↑ "Safety (MSDS) data for glycine". The Physical and Theoretical Chemistry Laboratory Oxford University. 2005. Retrieved 2006-11-01.
- ↑ Halson SL (2014). "Sleep in elite athletes and nutritional interventions to enhance sleep". Sports Med. 44 Suppl 1: S13–23. doi:10.1007/s40279-014-0147-0. PMC 4008810. PMID 24791913.
Glycine (a non-essential amino acid) functions as an inhibitory neurotransmitter in the central nervous system and also acts as a co-agonist of glutamate receptors. In a Japanese study [73], glycine has been shown to improve subjective sleep. Yamadera et al. [74] also reported shorter sleep-onset latencies measured by polysomnography. The authors suggested that potential mechanisms involve increased vasodilation and thus lowering of core temperature and increased extracellular 5-HT release in the prefrontal cortex of rats [74]
- ↑ Yamadera W, Inagawa K, Chiba S, Bannai M, Takahashi M, Nakayama K (2007). "Glycine ingestion improves subjective sleep quality in human volunteers, correlating with polysomnographic changes". Sleep and Biological rhythms 5 (2): 126–131. doi:10.1111/j.1479-8425.2007.00262.x.
- ↑ Coyle JT, G Tsai (2004). "The NMDA receptor glycine modulatory site: a therapeutic target for improving cognition and reducing negative symptoms in schizophrenia". Psychopharmacology 174: 32–28. doi:10.1007/s00213-003-1709-2.
- ↑ "Glycine From Japan and Korea" (PDF). Retrieved 2014-06-13.
- 1 2
- ↑ "Notice of Preliminary Determination of Sales at Less Than Fair Value: Glycine From India" Federal Register 72 (7 November 2007): 62827.
- ↑ Hashizume, O.; Ohnishi, S.; Mito, T.; Shimizu, A.; Iashikawa, K.; Nakada, K.; Soda, M.; Mano, H.; Togayachi, S.; Miyoshi, H.; Okita, K.; Hayashi, J. I. (2015). "Epigenetic regulation of the nuclear-coded GCAT and SHMT2 genes confers human age-associated mitochondrial respiration defects". Scientific Reports 5: 10434. doi:10.1038/srep10434.
- ↑ http://www.sciencedaily.com/releases/2015/05/150526085138.htm
- ↑ Snyder LE, Lovas FJ, Hollis JM, et al. (2005). "A rigorous attempt to verify interstellar glycine". Astrophys J 619 (2): 914–930. arXiv:astro-ph/0410335. Bibcode:2005ApJ...619..914S. doi:10.1086/426677.
- ↑ Staff. "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius 27 March 2008 - Science Daily". Retrieved 2008-09-16.
- ↑ Kvenvolden, Keith A.; Lawless, James; Pering, Katherine; Peterson, Etta; Flores, Jose; Ponnamperuma, Cyril; Kaplan, Isaac R.; Moore, Carleton (1970). "Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite". Nature 228 (5275): 923–926. Bibcode:1970Natur.228..923K. doi:10.1038/228923a0. PMID 5482102.
- ↑ Reuters (18 August 2009). "Building block of life found on comet - Thomson Reuters 2009". Retrieved 2009-08-18.
Further reading
On attempts to detect glycine in interstellar medium
- Kuan YJ, Charnley SB, Huang HC, et al. (2003). "Interstellar glycine". Astrophys J 593 (2): 848–867. Bibcode:2003ApJ...593..848K. doi:10.1086/375637.
- Rachel Nowak. "Amino acid found in deep space - 18 July 2002 - New Scientist". Retrieved 2007-07-01.
External links
| Wikimedia Commons has media related to Glycine. |
- Glycine MS Spectrum
- Glycine at PDRHealth.com
- Glycine cleavage system
- Glycine Therapy - A New Direction for Schizophrenia Treatment?
- "Organic Molecule, Amino Acid-Like, Found In Constellation Sagittarius". ScienceDaily. 27 March 2008.
- Guochuan E. Tsai (1 December 2008). "A New Class of Antipsychotic Drugs: Enhancing Neurotransmission Mediated by NMDA Receptors". Psychiatric Times 25 (14).
- ChemSub Online (Glycine).
- NASA scientists have discovered glycine, a fundamental building block of life, in samples of comet Wild 2 returned by NASA's Stardust spacecraft.
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
![K=\mathrm{ \frac {[H_3N^+CH_2CO_2^-]} {[H_2NCH_2CO_2H]}}](../I/m/69d940c4231de2dd0fcc73ae6b943bd7.png)

