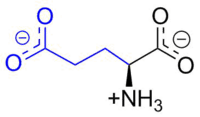
Glutamic acid
 | |
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
2-Aminopentanedioic acid | |
| Other names
2-Aminoglutaric acid | |
| Identifiers | |
| 56-86-0 (L isomer) 617-65-2 (D/L racemate) | |
| ChEBI | CHEBI:18237 |
| ChEMBL | ChEMBL276389. |
| ChemSpider | 591 |
| Jmol interactive 3D | Image |
| KEGG | D0434 |
| UNII | 61LJO5I15S |
| |
| |
| Properties | |
| C5H9NO4 | |
| Molar mass | 147.13 g·mol−1 |
| Appearance | white crystalline powder |
| Density | 1.4601 (20 °C) |
| Melting point | 199 °C (390 °F; 472 K) decomposes |
| 7.5 g/L (20 °C)[1] | |
| Solubility | 0.00035g/100g ethanol (25 °C)[2] |
| Acidity (pKa) | 2.1, 4.07, 9.47 [3] |
| Hazards | |
| Safety data sheet | See: data page |
| NFPA 704 | |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| | |
| Infobox references | |
Glutamic acid (abbreviated as Glu or E; encoded by the codons GAA or GAG) is an ɑ-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated -+NH3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated –COO- form under biological conditions), and a side chain carboxylic acid, classifying it as a polar negatively charged (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it.
In neuroscience, its carboxylate anion glutamate is an important excitatory neurotransmitter that plays the principal role in neural activation.[4]
Chemistry
The side chain carboxylic acid functional group has a pKa of 4.1 and therefore exists almost entirely in its negatively charged deprotonated carboxylate form at pH values greater than 4.1; therefore, it is negatively charged at physiological pH ranging from 7.35 to 7.45.
History
Although they occur naturally in many foods, the flavor contributions made by glutamic acid and other amino acids were only scientifically identified early in the twentieth century. The substance was discovered and identified in the year 1866, by the German chemist Karl Heinrich Ritthausen who treated wheat gluten (for which it was named) with sulfuric acid.[5] In 1908 Japanese researcher Kikunae Ikeda of the Tokyo Imperial University identified brown crystals left behind after the evaporation of a large amount of kombu broth as glutamic acid. These crystals, when tasted, reproduced the ineffable but undeniable flavor he detected in many foods, most especially in seaweed. Professor Ikeda termed this flavor umami. He then patented a method of mass-producing a crystalline salt of glutamic acid, monosodium glutamate.[6][7]
Biosynthesis
| Reactants | Products | Enzymes |
|---|---|---|
| Glutamine + H2O | → Glu + NH3 | GLS, GLS2 |
| NAcGlu + H2O | → Glu + Acetate | N-acetyl-glutamate synthase |
| α-ketoglutarate + NADPH + NH4+ | → Glu + NADP+ + H2O | GLUD1, GLUD2[8] |
| α-ketoglutarate + α-amino acid | → Glu + α-keto acid | transaminase |
| 1-Pyrroline-5-carboxylate + NAD+ + H2O | → Glu + NADH | ALDH4A1 |
| N-formimino-L-glutamate + FH4 | → Glu + 5-formimino-FH4 | FTCD |
| NAAG | → Glu + NAA | GCPII |
Function and uses
Metabolism
Glutamate is a key compound in cellular metabolism. In humans, dietary proteins are broken down by digestion into amino acids, which serve as metabolic fuel for other functional roles in the body. A key process in amino acid degradation is transamination, in which the amino group of an amino acid is transferred to an α-ketoacid, typically catalysed by a transaminase. The reaction can be generalised as such:
- R1-amino acid + R2-α-ketoacid ⇌ R1-α-ketoacid + R2-amino acid
A very common α-keto acid is α-ketoglutarate, an intermediate in the citric acid cycle. Transamination of α-ketoglutarate gives glutamate. The resulting α-ketoacid product is often a useful one as well, which can contribute as fuel or as a substrate for further metabolism processes. Examples are as follows:
- Aspartate + α-ketoglutarate ⇌ oxaloacetate + glutamate
Both pyruvate and oxaloacetate are key components of cellular metabolism, contributing as substrates or intermediates in fundamental processes such as glycolysis, gluconeogenesis, and the citric acid cycle.
Glutamate also plays an important role in the body's disposal of excess or waste nitrogen. Glutamate undergoes deamination, an oxidative reaction catalysed by glutamate dehydrogenase,[8] as follows:
Ammonia (as ammonium) is then excreted predominantly as urea, synthesised in the liver. Transamination can thus be linked to deamination, effectively allowing nitrogen from the amine groups of amino acids to be removed, via glutamate as an intermediate, and finally excreted from the body in the form of urea.
Glutamate is also a neurotransmitter (see below), which makes it one of the most abundant molecules in the brain. Malignant brain tumors known as glioma or glioblastoma exploit this phenomenon by using glutamate as an energy source, especially when these mutations become more dependent on glutamate due to mutations in the gene IDH1.[9][10]
Neurotransmitter
Glutamate is the most abundant excitatory neurotransmitter in the vertebrate nervous system.[11] At chemical synapses, glutamate is stored in vesicles. Nerve impulses trigger release of glutamate from the presynaptic cell. Glutamate acts on ionotropic and metabotropic (G-protein coupled) receptors.[11] In the opposing postsynaptic cell, glutamate receptors, such as the NMDA receptor or the AMPA receptor, bind glutamate and are activated. Because of its role in synaptic plasticity, glutamate is involved in cognitive functions such as learning and memory in the brain.[12] The form of plasticity known as long-term potentiation takes place at glutamatergic synapses in the hippocampus, neocortex, and other parts of the brain. Glutamate works not only as a point-to-point transmitter, but also through spill-over synaptic crosstalk between synapses in which summation of glutamate released from a neighboring synapse creates extrasynaptic signaling/volume transmission.[13] In addition, glutamate plays important roles in the regulation of growth cones and synaptogenesis during brain development as originally described by Mark Mattson.
Brain nonsynaptic glutamatergic signaling circuits
Extracellular glutamate in Drosophila brains has been found to regulate postsynaptic glutamate receptor clustering, via a process involving receptor desensitization.[14] A gene expressed in glial cells actively transports glutamate into the extracellular space,[14] while, in the nucleus accumbens-stimulating group II metabotropic glutamate receptors, this gene was found to reduce extracellular glutamate levels.[15] This raises the possibility that this extracellular glutamate plays an "endocrine-like" role as part of a larger homeostatic system.
GABA precursor
Glutamate also serves as the precursor for the synthesis of the inhibitory gamma-aminobutyric acid (GABA) in GABA-ergic neurons. This reaction is catalyzed by glutamate decarboxylase (GAD), which is most abundant in the cerebellum and pancreas.
Stiff-man syndrome is a neurologic disorder caused by anti-GAD antibodies, leading to a decrease in GABA synthesis and, therefore, impaired motor function such as muscle stiffness and spasm. Since the pancreas has abundant GAD, a direct immunological destruction occurs in the pancreas and the patients will have diabetes mellitus.
Flavor enhancer
Glutamic acid, being a constituent of protein, is present in every food that contains protein, but it can only be tasted when it is present in an unbound form. Significant amounts of free glutamic acid are present in a wide variety of foods, including cheese and soy sauce, and is responsible for umami, one of the five basic tastes of the human sense of taste. Glutamic acid is often used as a food additive and flavor enhancer in the form of its salt, known as monosodium glutamate (MSG).
Nutrient
All meats, poultry, fish, eggs, dairy products, and kombu are excellent sources of glutamic acid. Some protein-rich plant foods also serve as sources. 30% to 35% of the protein in wheat is glutamic acid. Ninety-five percent of the dietary glutamate is metabolized by intestinal cells in a first pass.[16]
Plant growth
Auxigro is a plant growth preparation that contains 30% glutamic acid.
NMR spectroscopy
In recent years, there has been much research into the use of residual dipolar coupling (RDC) in nuclear magnetic resonance spectroscopy (NMR). A glutamic acid derivative, poly-γ-benzyl-L-glutamate (PBLG), is often used as an alignment medium to control the scale of the dipolar interactions observed.[17]
Pharmacology
The drug phencyclidine (more commonly known as PCP) antagonizes glutamic acid non-competitively at the NMDA receptor. For the same reasons, dextromethorphan and ketamine also have strong dissociative and hallucinogenic effects. Acute infusion of the drug LY354740 (also known as eglumegad, an agonist of the metabotropic glutamate receptors 2 and 3) resulted in a marked diminution of yohimbine-induced stress response in bonnet macaques (Macaca radiata); chronic oral administration of LY354740 in those animals led to markedly reduced baseline cortisol levels (approximately 50 percent) in comparison to untreated control subjects.[18] LY354740 has also been demonstrated to act on the metabotropic glutamate receptor 3 (GRM3) of human adrenocortical cells, downregulating aldosterone synthase, CYP11B1, and the production of adrenal steroids (i.e. aldosterone and cortisol).[19] Glutamate does not easily pass the blood brain barrier, but, instead, is transported by a high-affinity transport system.[20] It can also be converted into glutamine.
See also
References
- ↑ http://www.chemicalbook.com/ProductChemicalPropertiesCB4355560_EN.htm
- ↑ Belitz, H.-D; Grosch, Werner; Schieberle, Peter (2009-02-27). "Food Chemistry". ISBN 9783540699330.
- ↑ http://www.cem.msu.edu/~cem252/sp97/ch24/ch24aa.html
- ↑ Robert Sapolsky (2005). "Biology and Human Behavior: The Neurological Origins of Individuality, 2nd edition". The Teaching Company.
see pages 19 and 20 of Guide Book
- ↑ R.H.A. Plimmer (1912) [1908]. R.H.A. Plimmer & F.G. Hopkins, ed. The Chemical Constitution of the Protein. Monographs on biochemistry. Part I. Analysis (2nd ed.). London: Longmans, Green and Co. p. 114. Retrieved June 3, 2012.
- ↑ Renton, Alex (2005-07-10). "If MSG is so bad for you, why doesn't everyone in Asia have a headache?". The Guardian. Retrieved 2008-11-21.
- ↑ "Kikunae Ikeda Sodium Glutamate". Japan Patent Office. 2002-10-07. Retrieved 2008-11-21.
- 1 2 Grabowska, A.; Nowicki, M.; Kwinta, J. (2011). "Glutamate dehydrogenase of the germinating triticale seeds: Gene expression, activity distribution and kinetic characteristics". Acta Physiologiae Plantarum 33 (5): 1981–1990. doi:10.1007/s11738-011-0801-1.
- ↑ van Lith, SA; Navis, AC; Verrijp, K; Niclou, SP; Bjerkvig, R; Wesseling, P; Tops, B; Molenaar, R; van Noorden, CJ; Leenders, WP (August 2014). "Glutamate as chemotactic fuel for diffuse glioma cells: are they glutamate suckers?". Biochimica et Biophysica Acta 1846 (1): 66–74. doi:10.1016/j.bbcan.2014.04.004. PMID 24747768.
- ↑ van Lith, SA; Molenaar, R; van Noorden, CJ; Leenders, WP (December 2014). "Tumor cells in search for glutamate: an alternative explanation for increased invasiveness of IDH1 mutant gliomas.". Neuro-oncology 16 (12): 1669–70. doi:10.1093/neuonc/nou152. PMID 25074540.
- 1 2 Meldrum, B. S. (2000). "Glutamate as a neurotransmitter in the brain: Review of physiology and pathology". The Journal of Nutrition 130 (4S Suppl): 1007S–1015S. PMID 10736372.
- ↑ McEntee, W. J.; Crook, T. H. (1993). "Glutamate: Its role in learning, memory, and the aging brain". Psychopharmacology 111 (4): 391–401. doi:10.1007/BF02253527. PMID 7870979.
- ↑ Okubo, Y.; Sekiya, H.; Namiki, S.; Sakamoto, H.; Iinuma, S.; Yamasaki, M.; Watanabe, M.; Hirose, K.; Iino, M. (2010). "Imaging extrasynaptic glutamate dynamics in the brain". Proceedings of the National Academy of Sciences 107 (14): 6526–6531. doi:10.1073/pnas.0913154107.
- 1 2 Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE (2007). "Nonvesicular Release of Glutamate by Glial xCT Transporters Suppresses Glutamate Receptor Clustering In Vivo". Journal of Neuroscience 27 (1): 111–123. doi:10.1523/JNEUROSCI.4770-06.2007. PMC 2193629. PMID 17202478.
- ↑ Zheng Xi, Baker DA, Shen H, Carson DS, Kalivas PW (2002). "Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens". Journal of Pharmacology and Experimental Therapeutics 300 (1): 162–171. doi:10.1124/jpet.300.1.162. PMID 11752112.
- ↑ Reeds, P.J.; et al. (1 April 2000). "Intestinal glutamate metabolism". Journal of Nutrition 130 (4s): 978S–982S. PMID 10736365.
- ↑ C. M. Thiele, Concepts Magn. Reson. A, 2007, 30A, 65-80
- ↑ Coplan JD, Mathew SJ, Smith EL, Trost RC, Scharf BA, Martinez J, Gorman JM, Monn JA, Schoepp DD, Rosenblum LA (July 2001). "Effects of LY354740, a novel glutamatergic metabotropic agonist, on nonhuman primate hypothalamic-pituitary-adrenal axis and noradrenergic function.". CNS Spectr. 6 (7): 607–12, 617. PMID 15573025.
- ↑ Felizola SJA, Nakamura Y, Satoh F, Morimoto R, Kikuchi K, Nakamura T, Hozawa A, Wang L, Onodera Y, Ise K, McNamara KM, Midorikawa S, Suzuki S, Sasano H (January 2014). "Glutamate receptors and the regulation of steroidogenesis in the human adrenal gland: The metabotropic pathway.". Molecular and Cellular Endocrinology 382 (1): 170–177. doi:10.1016/j.mce.2013.09.025. PMID 24080311.
- ↑ Smith QR (April 2000). "Transport of glutamate and other amino acids at the blood–brain barrier". J. Nutr. 130 (4S Suppl): 1016S–22S. PMID 10736373.
External links
| Look up glutamic acid in Wiktionary, the free dictionary. |
Further reading
| Wikimedia Commons has media related to Glutamic acid. |
- Nelson, David L.; Cox, Michael M. (2005), Principles of Biochemistry (4th ed.), New York: W. H. Freeman, ISBN 0-7167-4339-6
| ||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||
|

