Calcium bisulfite
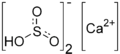 | |
 | |
| Names | |
|---|---|
| IUPAC name
Calcium hydrogen sulfite | |
| Other names
Calcium bisulphite E227 | |
| Identifiers | |
| 13780-03-5 | |
| ChemSpider | 24475 |
| Jmol interactive 3D | Image |
| PubChem | 26268 |
| RTECS number | EV9294500 |
| |
| |
| Properties | |
| Ca(HSO3)2 | |
| Molar mass | 202.22 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Calcium bisulfite (calcium bisulphite) is an inorganic compound which is the salt of a calcium cation and a bisulfite anion. It may be prepared by reacting lime with an excess of sulfurous acid, essentially a mixture of sulfur dioxide and water. It is a weak reducing agent, as is sulfur dioxide, sulfites, and any other compound containing sulfur in the +4 oxidation state. As a food additive it is used as a preservative under the E number E227. Calcium bisulfite is an acid salt and behaves like an acid in aqueous solution.
See also
This article is issued from Wikipedia - version of the Wednesday, November 18, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.