Triamterene
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
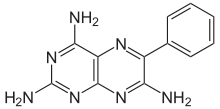
6-phenylpteridine-2,4,7-triamine | |
| Clinical data | |
| Trade names | Dyrenium, Dyazide, Maxzide |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682337 |
| Pregnancy category |
|
| Legal status | |
| Routes of administration | oral |
| Pharmacokinetic data | |
| Bioavailability | 30-70% |
| Protein binding | 67% |
| Metabolism | conjugated to hydroxytriamterene |
| Biological half-life | 1-2 hours, active metabolite 3 hours |
| Excretion | renal <50%, 21% unchanged |
| Identifiers | |
| CAS Number |
396-01-0 |
| ATC code | C03DB02 |
| PubChem | CID 5546 |
| IUPHAR/BPS | 4329 |
| DrugBank |
DB00384 |
| ChemSpider |
5345 |
| UNII |
WS821Z52LQ |
| KEGG |
D00386 |
| ChEMBL |
CHEMBL585 |
| Chemical data | |
| Formula | C12H11N7 |
| Molar mass | 253.263 g/mol |
| |
| | |
Triamterene (trade name Dyrenium) is a potassium-sparing diuretic used in combination with thiazide diuretics for the treatment of hypertension and edema. In combination with hydrochlorothiazide, it is marketed under the names Maxzide and Dyazide.
Mechanism of action
Triamterene directly blocks the epithelial sodium channel[1] (ENaC) on the lumen side of the kidney collecting tubule. Other diuretics cause a decrease in the sodium concentration of the forming urine due to the entry of sodium into the cell via the ENaC, and the concomitant exit of potassium from the principal cell into the forming urine. Blocking ENaC prevents this from happening. Amiloride works in the same way. Sodium channel blockers directly inhibit the entry of sodium into the sodium channels.
Side effects
Common side effects may include a depletion of sodium, folic acid and calcium, nausea, vomiting, diarrhea, headache, dizziness, fatigue, and dry mouth. Serious side effects may include heart palpitations, tingling/numbness, fever, chills, sore throat, rash, and back pain. Triamterene can also cause kidney stones through direct crystallization or by seeding calcium oxalate stones. Triamterene is best avoided in patients with chronic kidney disease due to the possibility of hyperkalemia. People using this drug should use salt substitute cautiously.[2]
Triamterene may impart a blue fluorescent color to the urine.
Caution with certain disease states
Diabetes: Use with caution in patients with prediabetes or diabetes mellitus as there may be a change in glucose control.
Hepatic impairment: Use with caution in patients with severe hepatic dysfunction; in cirrhosis, avoid electrolyte and acid/base imbalances that might lead to hepatic encephalopathy.
Kidney stones: Use with caution in patients with kidney stones.
Use should be avoided if the creatinine clearance is less than 10 ml/minute.
With hydrochlorothiazide
Triamterene is commonly prepared in combination with hydrochlorothiazide for treatment of hypertension (high blood pressure) and edema (water retention). This combination is in a class of medications called diuretics or 'water pills', and causes the kidneys to get rid of the body's unneeded water and sodium through the urine.[3] Dyazide is marketed by GlaxoSmithKline and Maxzide is marketed by Mylan.
Use in Ménière's disease
While there is a lack of randomized controlled trials evaluating the use of triamterene in the treatment of Ménière's disease, the typical treatment is 37.5 mg of triamterene with 25 mg of hydrochlorothiazide 1–2 capsules daily.[4][5] This recommendation was given a Strength of Recommendation Taxonomy (SORT) grade of C.
References
- ↑ Busch AE; Suessbrich H; Kunzelmann K; et al. (September 1996). "Blockade of epithelial Na+ channels by triamterenes - underlying mechanisms and molecular basis". Pflugers Arch. 432 (5): 760–6. doi:10.1007/s004240050196. PMID 8772124.
- ↑ LoSalt Advisory Statement (PDF) Archived December 10, 2005 at the Wayback Machine
- ↑ "Triamterene and Hydrochlorothiazide". MedlinePlus. U.S. National Library of Medicine. National Institutes of Health. September 1, 2008.
- ↑ Swartz R; Longwell P (March 2005). "Treatment of vertigo" (PDF). Am Fam Physician 71 (6): 1115–22. PMID 15791890.
- ↑ Sloane PD; Coeytaux RR; Beck RS; Dallara J (May 2001). "Dizziness: state of the science". Ann. Intern. Med. 134 (9 Pt 2): 823–32. doi:10.7326/0003-4819-134-9_Part_2-200105011-00005. PMID 11346317.
External links
- Dyazide Prescribing Information GlaxoSmithKline
| ||||||||||||||||||||||||||||||||||||||||||||||