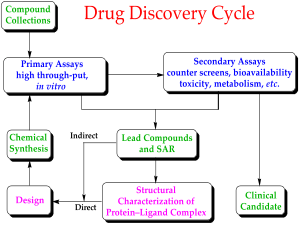
Drug development
Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes pre-clinical research on microorganisms and animals, clinical trials on humans, and may include the step of obtaining regulatory approval to market the drug.
New chemical entity development
Broadly, the process of drug development can be divided into pre-clinical and clinical work.
Pre-clinical
New chemical entities (NCEs, also known as new molecular entities or NMEs) are compounds which emerge from the process of drug discovery. These will have promising activity against a particular biological target thought to be important in disease; however, little will be known about the safety, toxicity, pharmacokinetics and metabolism of this NCE in humans. It is the function of drug development to assess all of these parameters prior to human clinical trials. A further major objective of drug development is to make a recommendation of the dose and schedule to be used the first time an NCE is used in a human clinical trial ("first-in-man" [FIM] or First Human Dose [FHD]).
In addition, drug development is required to establish the physicochemical properties of the NCE: its chemical makeup, stability, solubility. The process by which the chemical is made will be optimized so that from being made at the bench on a milligram scale by a medicinal chemist, it can be manufactured on the kilogram and then on the ton scale. It will be further examined for its suitability to be made into capsules, tablets, aerosol, intramuscular injectable, subcutaneous injectable, or intravenous formulations. Together these processes are known in preclinical development as Chemistry, Manufacturing and Control (CMC).
Many aspects of drug development are focused on satisfying the regulatory requirements of drug licensing authorities. These generally constitute a number of tests designed to determine the major toxicities of a novel compound prior to first use in man. It is a legal requirement that an assessment of major organ toxicity be performed (effects on the heart and lungs, brain, kidney, liver and digestive system), as well as effects on other parts of the body that might be affected by the drug (e.g. the skin if the new drug is to be delivered through the skin). While, increasingly, these tests can be made using in vitro methods (e.g. with isolated cells), many tests can only be made by using experimental animals, since it is only in an intact organism that the complex interplay of metabolism and drug exposure on toxicity can be examined.
The information is gathered from this pre-clinical testing, as well as information on CMC, and submitted to regulatory authorities (in the US, to the FDA), as an Investigational New Drug application or IND. If the IND is approved, development moves to the clinical phase.
Clinical phase
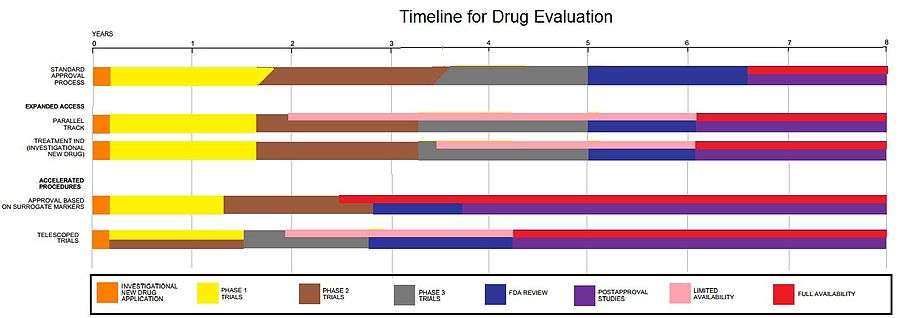
Clinical trials involve three or four steps:[1]
- Phase I trials, usually in healthy volunteers, determine safety and dosing.
- Phase II trials are used to get an initial reading of efficacy and further explore safety in small numbers of sick patients.
- Phase III trials are large, pivotal trials to determine safety and efficacy in sufficiently large numbers of patients.
- Phase IV trials are post-approval trials that are sometimes a condition attached by the FDA, also called post-market surveillance studies.
The process of drug development doesn't stop once an NCE begins human clinical trials. In addition to the tests required to move a novel drug into the clinic for the first time it is also important to ensure that long-term or chronic toxicities are determined, as well as effects on systems not previously monitored (fertility, reproduction, immune system, etc.). The compound will also be tested for its capability to cause cancer (carcinogenicity testing).
If a compound emerges from these tests with an acceptable toxicity and safety profile, and it can further be demonstrated to have the desired effect in clinical trials, then it can be submitted for marketing approval in the various countries where it will be sold. In the US, this process is called a New Drug Application or NDA. Most NCEs, however, fail during drug development, either because they have some unacceptable toxicity, or because they simply do not work in clinical trials.
Cost
The full cost of bringing a new drug (i.e. a drug that is a new chemical entity) to market – from discovery through clinical trials to approval – is complex and controversial; typically it is hundreds of millions or billions of U.S. dollars. One element of the complexity is that the much-publicized final numbers often do not include just the simple out-of-pocket expenses, but also include "capital costs", which are included to take into account the long time period (often at least ten years) during which the out-of-pocket costs are expended; additionally it is often not stated whether a given figure includes the capitalized cost or comprises only out-of-pocket expenses. Another element of complexity is that all estimates are based on confidential information owned by drug companies, released by them voluntarily. There is currently no way to validate these numbers. The numbers are controversial, as drug companies use them to justify the prices of their drugs and various advocates for lower drug prices have challenged them. The controversy is not only between "high" and "low" – the numbers also vary greatly at the high end.
A study published by Steve Paul et al. in 2010 in Nature Reviews: Drug Discovery compares many of the studies, provides both capitalized and out-of-pocket costs for each, and lays out the assumptions each makes: see Supplemental Box 2.[2] The authors offer their own estimate of the capitalized cost as being ~$1.8B, with out-of-pocket costs of ~$870M.
Studies published by diMasi et al. in 2003, report an average pre-tax, capitalized cost of approximately $800 million to bring one of the drugs from the study to market. Also, this $800 million figure includes opportunity costs of $400 million.[3] A study published in 2006 estimates that costs vary from around $500 million to $2 billion depending on the therapy or the developing firm.[4] A study published in 2010 in the journal Health Economics, including an author from the US Federal Trade Commission, was critical of the methods used by diMasi et al. but came up with a higher estimate of ~$1.2 billion.[5]
Valuation
The nature of a drug development project is characterised by high attrition rates, large capital expenditures, and long timelines. This makes the valuation of such projects and companies a challenging task. Not all valuation methods can cope with these particularities. The most commonly used valuation methods are risk-adjusted net present value (rNPV), decision trees, real options, or comparables.
The most important value drivers are the cost of capital or discount rate that is used, phase attributes such as duration, success rates, and costs, and the forecasted sales, including cost of goods and marketing and sales expenses. Less objective aspects like quality of the management or novelty of the technology should be reflected in the cash flows estimation.[6][7]
Success rate
Candidates for a new drug to treat a disease might theoretically include from 5,000 to 10,000 chemical compounds. On average about 250 of these will show sufficient promise for further evaluation using laboratory tests, mice and other test animals. Typically, about ten of these will qualify for tests on humans.[8] A study conducted by the Tufts Center for the Study of Drug Development covering the 1980s and 1990s found that only 21.5 percent of drugs that start phase I trials are eventually approved for marketing.[9] The high failure rates associated with pharmaceutical development are referred to as the "attrition rate" problem. Careful decision making during drug development is essential to avoid costly failures.[10] In many cases, intelligent programme and clinical trial design can prevent false negative results. Well designed dose-finding studies and comparisons against both a placebo and a gold-standard treatment arm play a major role in achieving reliable data.[11]
Novel initiatives to boost drug development
Novel initiatives include partnering between governmental organizations and industry. The world's largest such initiative is the Innovative Medicines Initiative (IMI), and examples of major national initiatives are Top Institute Pharma in the Netherlands and Biopeople in Denmark. In 2004, the FDA created the “Critical Path Initiative” (CPI) project to guide the new drug development process.[12] Some researchers are testing artificial organs on microchips meant to provide more accurate simulations than mice.
See also
- Clinical trial
- Council for International Organizations of Medical Sciences
- Drug design
- Drug discovery
- Drug repositioning
- Generic drug
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use, a consensus between the U.S. Food and Drug Administration (FDA), EU, and Japan.
- Investigational New Drug, FDA application/permission to start clinical trials
- List of pharmaceutical companies
- Pre-clinical development
- Biopeople
References
- ↑ Ciociola AA; et al. (May 2014). "How drugs are developed and approved by the FDA: current process and future directions". Am J Gastroenterol 109 (5): 620–3. doi:10.1038/ajg.2013.407. PMID 24796999.
- ↑ How to improve R&D productivity: the pharmaceutical industry's grand challenge : Article : Nature Reviews Drug Discovery
- ↑ DiMasi, Joseph A.; Hansen, Ronald W.; Grabowski, Henry G. (March 2003). "The price of innovation: new estimates of drug development costs". Journal of Health Economics 22 (2): 151–185. doi:10.1016/S0167-6296(02)00126-1. PMID 12606142.
- ↑ Adams C, Brantner V (2006). "Estimating the cost of new drug development: is it really 802 million dollars?". Health Aff (Millwood) 25 (2): 420–8. doi:10.1377/hlthaff.25.2.420. PMID 16522582.
- ↑ Adams, Christopher Paul; Brantner, Van Vu (February 2010). "Spending on new drug development". Health Economics 19 (2): 130–141. doi:10.1002/hec.1454. PMID 19247981.
- ↑ Boris Bogdan and Ralph Villiger, "Valuation in Life Sciences. A Practical Guide", 2008, 2nd edition, Springer Verlag.
- ↑ Nielsen, Nicolaj Hoejer "Financial valuation methods for biotechnology", 2010.
- ↑ Stratmann, Dr. H.G. (September 2010). "Bad Medicine: When Medical Research Goes Wrong". Analog Science Fiction and Fact CXXX (9): 20.
- ↑ "R&D costs are on the rise". Medical Marketing and Media 38 (6): 14. June 1, 2003.
- ↑ Wang Y. (2012). "Extracting Knowledge from Failed Development Programmes". Pharm Med 26 (2): 91–96. doi:10.1007/BF03256897.
- ↑ Herschel, M. (2012). "Portfolio Decisions in Early Development: Don't Throw Out the Baby with the Bathwater". Pharm Med 26 (2): 77–84. doi:10.1007/BF03256895.
- ↑ http://www.irjpas.com/zip.php?file=File_Folder/IRJPAS%203%283%2929-37.pdf&id=235&quat=3
External links
- CDER Drug and Biologic Approval Reports
- International Union of Basic and Clinical Pharmacology
- Henninger, Daniel (2002). "Drug Lag". In David R. Henderson (ed.). Concise Encyclopedia of Economics (1st ed.). Library of Economics and Liberty. OCLC 317650570, 50016270 and 163149563