Doxylamine
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
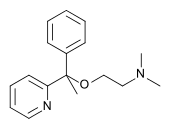
(RS)-N,N-dimethyl-2-(1-phenyl-1-pyridin-2-yl-ethoxy)-ethanamine | |
| Clinical data | |
| Trade names | Unisom |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682537 |
| Pregnancy category | |
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | Oral: 24.7%, Intranasal: 70.8%[1] |
| Metabolism | Hepatic (CYP3A4-meditated) |
| Biological half-life | 6–12 hours |
| Excretion | Urine (60%), feces (40%)[2] |
| Identifiers | |
| CAS Number |
469-21-6 |
| ATC code | R06AA09 |
| PubChem | CID 3162 |
| IUPHAR/BPS | 7171 |
| DrugBank |
DB00366 |
| ChemSpider |
3050 |
| UNII |
95QB77JKPL |
| KEGG |
D07878 |
| ChEBI |
CHEBI:51380 |
| ChEMBL |
CHEMBL1004 |
| Chemical data | |
| Formula | C17H22N2O |
| Molar mass | 270.369 g/mol |
| |
| |
| (verify) | |
Doxylamine[3] is a first-generation antihistamine, first reported in 1949.[4] It can be used by itself as a short-term sedative and in combination with other drugs to provide night-time allergy and cold relief. Doxylamine is also used in combination with the analgesics paracetamol (acetaminophen) and codeine as an analgesic/calmative preparation, e.g., can be taken in combination with pregabalin too, and is prescribed in combination with vitamin B6 (pyridoxine) to prevent morning sickness in pregnant women. Its fetal safety is “A” in Briggs’ Reference Guide to Foetal and Neonatal Risk.[5]
Indications
Doxylamine is a member of the ethanolamine class of antihistamines and has anti-allergy power superior to almost every other antihistamine on the market, with the exception of diphenhydramine (Benadryl); it is also the most effective over-the-counter sedative available in the United States and is more sedating than some prescription hypnotics.[6] One study found that doxylamine succinate was more effective than the barbiturate phenobarbital for use as a sedative.[6] The dosage required to induce sleep can be as low as 6.25 mg, but is usually effective in dosages of up to 25 mg. Higher doses are not recommended by the United States Food and Drug Administration, although single dosage recommendations of up to 50 mg are common in some countries, including Australia (where it is marketed under the names Restavit and Dozile), Russia (Valocordin-Doxylamine) and India (Vomentin).
A 2010 placebo-controlled, double-blind randomized controlled trial found the formulation of doxylamine and pyridoxine marketed as Diclegis to be effective in controlling nausea and vomiting due to pregnancy.[7]
Metabolites
The main metabolites are N-desmethyldoxylamine, N,N-didesmethyldoxylamine, and doxylamine N-oxide.[8][9]
Side effects
Doxylamine succinate is a potent anticholinergic and has a side-effect profile common to such drugs, including dry mouth, ataxia, urinary retention, drowsiness, memory problems, inability to concentrate, hallucinations, psychosis, and a marked increased sensitivity to external stimuli. Like many hypnotics, it should not be combined with other antihistamines, such as Zyrtec (cetirizine) or Benadryl (diphenhydramine), as this combination can increase the risk of serious side effects. Using doxylamine over a long period of time is not recommended. However, the drug is not addictive, and withdrawal effects are unlikely to be experienced with prolonged use.
Toxicity
Doxylamine succinate is generally safe for administration to healthy adults. Typical preparations that contain doxylamine range from 6.25 mg to 50 mg. The median lethal dose (LD50) is estimated to be 50–500 mg/kg in humans.[10] Symptoms of overdose may include dry mouth, dilated pupils, insomnia, night terrors, euphoria, hallucinations, seizures, rhabdomyolysis, and death.[11] Fatalities have been reported from doxylamine overdose. These have been characterized by coma, tonic-clonic (or grand mal) seizures and cardiorespiratory arrest. Children appear to be at a high risk for cardiorespiratory arrest. A toxic dose for children of more than 1.8 mg/kg has been reported. A 3-year old child died 18 hours after ingesting 1,000 mg doxylamine succinate.[2] Rarely, an overdose results in rhabdomyolysis and acute renal failure.[12]
Studies of doxylamine's carcinogenicity in mice and rats have produced positive results for both liver and thyroid cancer, especially in the mouse.[13] The carcinogenicity of the drug in humans is not well studied, and the IARC lists the drug as "not classifiable as to its carcinogenicity to humans".[14]
Formulations
Doxylamine is primarily used as the succinic acid salt, doxylamine succinate.
- It is the sedating ingredient of NyQuil (generally in combination with dextromethorphan and acetaminophen)
- In Commonwealth countries, such as Australia, Canada, South Africa and the United Kingdom, doxylamine is available prepared with paracetamol (acetaminophen) and codeine under the brand name Dolased, Propain Plus, Syndol (UK version no longer contains doxylamine as of 2015), or Mersyndol, as treatment for tension headache and other types of pain.
- Doxylamine succinate is used in general over-the-counter sleep-aids branded as Somnil (South Africa), Dozile, Donormyl (France, Russian Federation), Dormidina (Spain, Portugal), Restavit, Unisom-2 and Sleep Aid (generic, Australia).
- In the United States:
- doxylamine succinate is the active ingredient in the over-the-counter sleep-aid tablets branded as Unisom SleepTabs; however, all other Unisom products contain diphenhydramine hydrochloride instead.[15]
- doxylamine succinate and pyridoxine (Vitamin B6) are the ingredients of Diclegis, approved by the FDA in April 2013 as the only drug for morning sickness with a class A safety rating for pregnancy.[16]
- In Canada:
- doxylamine succinate was the active ingredient in the over-the-counter sleep-aid marketed as Unisom-2 tablets (no longer available). Unisom now solely markets products containing diphenhydramine hydrochloride as the active ingredient.[17]
- doxylamine succinate and pyridoxine (vitamin B6) are the ingredients of Diclectin, which is used to prevent morning sickness.
- It is also available in combination with vitamin B6 and folic acid under the brand name Evanorm (marketed by Ion Healthcare) .
See also
References
- ↑ Pelser, Andries; Müller, Douw G.; du Plessis, Jeanetta; du Preez, Jan L.; Goosen, Colleen (2002). "Comparative pharmacokinetics of single doses of doxylamine succinate following intranasal, oral and intravenous administration in rats". Biopharmaceutics & Drug Disposition 23 (6): 239–244. doi:10.1002/bdd.314. PMID 12214324.
- 1 2 Dozile. medsafe.govt.nz
- ↑ Cada, DJ; Demaris, K; Levien, TL; Baker, DE (October 2013). "Doxylamine succinate/pyridoxine hydrochloride.". Hospital pharmacy 48 (9): 762–6. doi:10.1310/hpj4809-762. PMC 3857125. PMID 24421551.
- ↑ Sperber, Nathan.; Papa, Domenick.; Schwenk, Erwin.; Sherlock, Margaret. (1949). "Pyridyl-Substituted Alkamine Ethers as Antihistaminic Agents". Journal of the American Chemical Society 71 (3): 887–890. doi:10.1021/ja01171a034. PMID 18113525.
- ↑ Briggs, Gerald G.; Freeman, Roger K.; Yaffe, Sumner J. (2008). Drugs in Pregnancy and Lactation: A Reference Guide to Fetal and Neonatal Risk. Lippincott Williams & Wilkins. doi:10.1258/om.2009.090002. ISBN 978-0-7817-7876-3.
- 1 2 Doxylamine. drugbank.ca
- ↑ Koren, Gideon; Clark, Shannon; Hankins, Gary D.V.; Caritis, Steve N.; Miodovnik, Menachem; Umans, Jason G.; Mattison, Donald R. (2010). "Effectiveness of delayed-release doxylamine and pyridoxine for nausea and vomiting of pregnancy: a randomized placebo controlled trial". American Journal of Obstetrics and Gynecology 203 (6): 571.e1–571.e7. doi:10.1016/j.ajog.2010.07.030. PMID 20843504.
- ↑ Holder, C. L.; Korfmacher, W. A.; Slikker Jr, W.; Thompson Jr, H. C.; Gosnell, A. B. (1985). "Mass spectral characterization of doxylamine and its rhesus monkey urinary metabolites". Biomedical mass spectrometry 12 (4): 151–158. doi:10.1002/bms.1200120403. PMID 2861861.
- ↑ rxlist.com, Khedezla.
- ↑ DOXYLAMINE SUCCINATE. hazard.com
- ↑ Syed, Husnain; Sumit Som; Nazia Khan; Wael Faltas (17 March 2009). "Doxylamine toxicity: seizure, rhabdomyolysis and false positive urine drug screen for methadone". BMJ Case Reports (BMJ Group) 2003 (90): 845. doi:10.1136/bcr.09.2008.0879. PMID 21686586.
- ↑ Leybishkis B, B; Fasseas P; Ryan KF. (2001). "Doxylamine overdose as a potential cause of rhabdomyolysis". American Journal of the Medical Sciences (Lippincott Williams & Wilkins) 322 (1): 48–9. doi:10.1097/00000441-200107000-00009. PMID 11465247.
- ↑ Doxylamine succinate (CAS 562-10-7). berkeley.edu
- ↑ DOXYLAMINE SUCCINATE. International Agency for Research on Cancer (IARC) - Summaries & Evaluations
- ↑ chattem.com, UNISOM Drug facts
- ↑ Slaughter, Shelley R.; Hearns-Stokes, Rhonda; van der Vlugt, Theresa; Joffe, Hylton V. (2014). "FDA Approval of Doxylamine–Pyridoxine Therapy for Use in Pregnancy". New England Journal of Medicine 370 (12): 1081–1083. doi:10.1056/NEJMp1316042. PMID 24645939.
- ↑ unisom.ca product page
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||