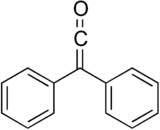
Diphenylketene
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2,2-Di(phenyl)ethenone | |
| Other names
Diphenylethenone | |
| Identifiers | |
| 525-06-4 | |
| ChemSpider | 109691 |
| Jmol interactive 3D | Image Image |
| PubChem | 123069 |
| |
| |
| Properties | |
| C14H10O | |
| Molar mass | 194.23 g·mol−1 |
| Appearance | Red-orange oil |
| Melting point | 8 to 9 °C (46 to 48 °F; 281 to 282 K) |
| Boiling point | 118 to 120 at 1mmHg |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diphenylketene is a chemical substance of the ketene family. Diphenylketene, like most disubstituted ketenes, is a red-orange oil at room temperature and pressure.
Reactivity
Diphenylketene can undergo attack from a host of nucleophiles, including alcohols, amines, and enolates with fairly slow rates. These rates can be increased in the presence of catalysts. At present the mechanism of attack is unknown, but work is underway to determine the exact mechanism.
Synthesis
Diphenylketene is produced by the elimination of hydrogen chloride from diphenylacetyl chloride in the presence of triethylamine.[1]

Spectroscopic information
1H NMR - Ar protons only (delta shifts between 7 and 8 ppm, showing 1 doublet and two triplets.
UV/vis - Lambda max at 267 nm
IR - charicteristic ketene abs. at 2100 cm−1
References
- ↑ Submitted by Edward C. Taylor Alexander McKillop, and George H. Hawks.
Checked by C. J. Michejda, D. D. von Riesen, R. W. Comnick, and Henry E. Baumgarten. (1972). "DIPHENYLKETENE [Ethenone, diphenyl-]". Organic Syntheses 52: 36. doi:10.15227/orgsyn.052.0036. line feed character in
|authors=at position 71 (help)