1,4-Dioxane
| |||
| Names | |||
|---|---|---|---|
| IUPAC names
1,4-Dioxane 1,4-Dioxacyclohexane | |||
| Other names
[1,4]Dioxane p-Dioxane [6]-crown-2 Diethylene dioxide Diethylene ether Dioxan | |||
| Identifiers | |||
| 123-91-1 | |||
| ChEBI | CHEBI:47032 | ||
| ChEMBL | ChEMBL453716 | ||
| ChemSpider | 29015 | ||
| DrugBank | DB03316 | ||
| EC Number | 204-661-8 | ||
| Jmol interactive 3D | Image | ||
| KEGG | C14440 | ||
| UNII | J8A3S10O7S | ||
| |||
| |||
| Properties | |||
| C4H8O2 | |||
| Molar mass | 88.11 g·mol−1 | ||
| Appearance | Colorless liquid[1] | ||
| Odor | mild, ether-like[1] | ||
| Density | 1.033 g/mL | ||
| Melting point | 11.8 °C (53.2 °F; 284.9 K) | ||
| Boiling point | 101.1 °C (214.0 °F; 374.2 K) | ||
| Miscible | |||
| Vapor pressure | 29 mmHg (20°C)[1] | ||
| Thermochemistry | |||
| Std molar entropy (S |
196.6 J·K−1·mol−1 | ||
| Std enthalpy of formation (ΔfH |
-354 kJ/mol | ||
| Std enthalpy of combustion (ΔcH |
-2363 kJ/mol | ||
| Hazards | |||
| Main hazards | Carcinogen[1] | ||
| EU classification (DSD) |
Flammable (F) Carc. Cat. 3 Irritant (Xn) | ||
| R-phrases | R11, R19, R36/37, R40, R66 | ||
| S-phrases | (S2), S9, S16, S36/37, S46 | ||
| NFPA 704 | |||
| Flash point | 12 °C (54 °F; 285 K) | ||
| Explosive limits | 2.0%-22%[1] | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (Median dose) |
5.7 g/kg (mouse, oral) 5.2 g/kg (rat, oral) 3.9 g/kg (guinea pig, oral) 7.6 g/kg (rabbit, dermal) | ||
| LC50 (Median concentration) |
10,109 ppm (mouse, 2 hr) 12,568 ppm (rat, 2 hr)[2] | ||
| LCLo (Lowest published) |
1000-3000 ppm (guinea pig, 3 hr) 12022 ppm (cat, 7 hr) 2085 ppm (mouse, 8 hr)[2] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 100 ppm (360 mg/m3) [skin][1] | ||
| REL (Recommended) |
Ca C 1 ppm (3.6 mg/m,sup>3) [30-minute][1] | ||
| IDLH (Immediate danger |
Ca [500 ppm][1] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
1,4-Dioxane, often simply called dioxane because the 1,2 and 1,3 isomers of dioxane are rare, is a heterocyclic organic compound. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. It is classified as an ether. Dioxane is used mainly as a stabilizer for the solvent trichloroethane. It is an occasionally used solvent for a variety of practical applications as well as in the laboratory.
Synthesis and structure
Dioxane is produced by the acid-catalysed dehydration of diethylene glycol, which in turn arises from the hydrolysis of ethylene oxide. The molecule is centrosymmetric, meaning that it adopts a chair conformation, typical of relatives of cyclohexane. The molecule is conformationally flexible, and the boat conformation is easily adopted, as required for chelation to metal cations. In 1985, the global production capacity for dioxane was between 11,000 and 14,000 tons.[3] In 1990, the total U.S. production volume of dioxane was between 10,500,000 and 18,300,000 pounds (5,250 to 9,150 tons).[4]
Dioxane has three isomers: in addition to 1,4-dioxane, 1,2-dioxane and 1,3-dioxane are also known.
Uses
Dioxane is primarily used as a stabilizer for 1,1,1-trichloroethane for storage and transport in aluminium containers. Normally aluminium is protected by a passivating oxide layer, but when these layers are disturbed, highly reactive metallic aluminium is exposed to the chlorocarbon. This aluminium reacts with 1,1,1-trichloroethane to give aluminium trichloride, which in turn catalyses the dehydrohalogenation of the remaining 1,1,1-trichloroethane to vinylidene chloride and hydrogen chloride. Reflecting its properties as a ligand, dioxane "poisons" the aluminum trichloride catalyst, by formation of an adduct.[3] Apart from its use as a stabilizer, dioxane is used in a variety of applications as a solvent, e.g. in inks and adhesives.
Solvent properties
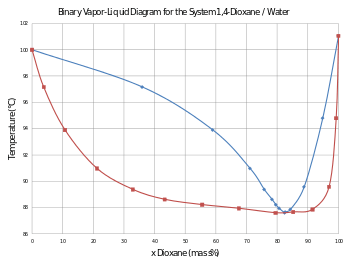
While diethyl ether is rather insoluble in water, dioxane is miscible and in fact is hygroscopic. This water miscibility is a favorable property for some industrial applications. At standard pressure, the mixture of water and dioxane in the ratio 17.9:82.1 by mass is a positive azeotrope that boils at 87.6 °C.[5] Dioxane is a versatile aprotic solvent. The oxygen atom is Lewis basic, so it is able to solvate many inorganic compounds. Because of its lower toxicity, it is substituted for tetrahydrofuran (THF) in some processes. However, it has a higher boiling point (101 °C versus 66 °C for THF), which is important when reactions are to be conducted at a higher temperature.
The oxygen centres are Lewis basic, and so dioxane serves as a chelating diether ligand. It reacts with Grignard reagents to precipitate the magnesium dihalide. In this way, dioxane is used to drive the Schlenk equilibrium.[3] Dimethylmagnesium is prepared in this manner:[6][7]
- 2 CH3MgBr + (C2H4O)2 → MgBr2(C2H4O)2 + (CH3)2Mg
Dioxane is used as an internal standard for proton NMR spectroscopy in D2O.[8]
Toxicology
Safety
Dioxane has an LD50 of 5170 mg/kg,[3] making it less acutely toxic than table salt (3000 mg/kg).[9] This compound is irritating to the eyes and respiratory tract. Exposure may cause damage to the central nervous system, liver and kidneys.[10] Accidental worker exposure to 1,4-dioxane has resulted in several deaths.[11] Dioxane is classified by the National Toxicology Program as "reasonably anticipated to be a human carcinogen".[12] It is also classified by the IARC as a Group 2B carcinogen: possibly carcinogenic to humans because it is a known carcinogen in other animals.[13] The U.S. Environmental Protection Agency classifies dioxane as a probable human carcinogen (having observed an increased incidence of cancer in controlled animal studies, but not in epidemiological studies of workers using the compound), and a known irritant (with a no-observed-adverse-effects level of 400 milligrams per cubic meter) at concentrations significantly higher than those found in commercial products.[14] Under Proposition 65, dioxane is classified in the U.S. State of California to cause cancer.[15] Animal studies in rats suggest that the greatest health risk is associated with inhalation of vapors in the pure form.[16][17][18]
Like some other ethers, dioxane combines with atmospheric oxygen upon prolonged exposure to air to form potentially explosive peroxides. Distillation of dioxanes concentrates these peroxides increasing the danger.
Environment
Dioxane has affected groundwater supplies in several areas. Dioxane at the level of 1 μg/L (~1 ppb) has been detected in many locations in the US.[4] In the State of New Hampshire alone in 2010 it had been found at 67 sites, ranging in concentration from 2 ppb to over 11,000 ppb. Thirty of these sites are solid waste landfills, most of which have been closed for years. It also has low toxicity to aquatic life and can be biodegraded via a number of pathways.[19] The problems are exacerbated since dioxane is highly soluble in water, does not readily bind to soils, and readily leaches to groundwater. It is also resistant to naturally occurring biodegradation processes. Due to these properties, a dioxane plume is often much larger (and further downgradient) than the associated solvent plume.
Cosmetics
As a byproduct of the ethoxylation process, a route to some ingredients found in cleansing and moisturizing products, dioxane can contaminate cosmetics and personal care products such as deodorants, shampoos, toothpastes and mouthwashes.[20][21] The ethoxylation process makes the cleansing agents, such as sodium lauryl sulfate, less abrasive and offers enhanced foaming characteristics. 1,4-Dioxane is found in small amounts in some cosmetics, a yet unregulated substance used in cosmetics in both China and the U.S.[22]
In 2008, testing sponsored by the U.S. Organic Consumers Association found dioxane in almost half of tested organic personal-care products.[23] Since 1979 the U.S. Food and Drug Administration (FDA) have conducted tests on cosmetic raw materials and finished products for the levels of 1,4-dioxane.[24] 1,4-Dioxane was present in ethoxylated raw ingredients at levels up to 1410 ppm, and at levels up to 279 ppm in off the shelf cosmetic products.[24] Levels of 1,4-dioxane exceeding 85 ppm in children's shampoos indicate that close monitoring of raw materials and finished products is warranted.[24] While the FDA encourages manufacturers to remove 1,4-dioxane, it is not required by federal law.[25]
See also
References
- 1 2 3 4 5 6 7 8 "NIOSH Pocket Guide to Chemical Hazards #0237". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 "Dioxane". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 4 Surprenant, Kenneth S. (2000). "Dioxane in Ullmann's Encyclopedia of Industrial Chemistry". doi:10.1002/14356007.a08_545.
- 1 2 "1, 4-Dioxane Fact Sheet: Support Document" (PDF). OPPT Chemical Fact Sheets. United States Environmental Protection Agency. February 1995. Retrieved 14 May 2010.
- ↑ Schneider, C. H.; Lynch, C. C.: The Ternary System: Dioxane-Ethanol-Water in J. Am. Chem. Soc., 1943, vol. 65, pp 1063–1066. doi:10.1021/ja01246a015
- ↑ Cope, Arthur C. (1935). "The Preparation of Dialkylmagnesium Compounds from Grignard Reagents". Journal of the American Chemical Society 57 (11): 2238. doi:10.1021/ja01314a059.
- ↑ Anteunis, M. (1962). "Studies of the Grignard Reaction. II. Kinetics of the Reaction of Dimethylmagnesium with Benzophenone and of Methylmagnesium Bromide-Magnesium Bromide with Pinacolone". The Journal of Organic Chemistry 27 (2): 596. doi:10.1021/jo01049a060.
- ↑ Shimizu, A.; Ikeguchi, M.; Sugai, S. (1994). "Appropriateness of DSS and TSP as internal references for 1H NMR studies of molten globule proteins in aqueous media". Journal of Biomolecular NMR 4 (6): 859. doi:10.1007/BF00398414.
- ↑ "NIH ToxNet Database".
- ↑ "International Chemical Safety Card". National Institute for Occupational Safety and Health. Retrieved 2 February 2006.
- ↑ "OPPT Chemical Fact Sheets 1,4-Dioxane (CAS No. 123-91-1)". United States Environmental Protection Agency. Retrieved 2 February 2006.
- ↑ "12th Report on Carcinogens". United States Department of Health and Human Services’ National Toxicology Program. Retrieved 11 July 2014.
- ↑ "IARC Monographs Volume 71" (PDF). International Agency for Research on Cancer. Retrieved 11 July 2014.
- ↑ 1,4-Dioxane (1,4-Diethyleneoxide). Hazard Summary. U.S. Environmental Protection Agency. Created in April 1992; Revised in January 2000. Fact Sheet
- ↑ "Chemicals Known to the State to Cause Cancer or Reproductive Toxicity" (PDF). Office of Environmental Health Hazard Assessment. 2 April 2010. Archived from the original (PDF) on 24 May 2010. Retrieved 14 December 2013.
1,4-Dioxane CAS#123-91-1 (Listed January 1, 1988)
- ↑ Kano, Hirokazu; Umeda, Yumi; Saito, Misae; Senoh, Hideki; Ohbayashi, Hisao; Aiso, Shigetoshi; Yamazaki, Kazunori; Nagano, Kasuke; Fukushima, Shoji (2008). "Thirteen-week oral toxicity of 1,4-dioxane in rats and mice". The Journal of toxicological sciences 33 (2): 141–53. doi:10.2131/jts.33.141. PMID 18544906.
- ↑ Kasai, T; Saito, M; Senoh, H; Umeda, Y; Aiso, S; Ohbayashi, H; Nishizawa, T; Nagano, K; Fukushima, S (2008). "Thirteen-week inhalation toxicity of 1,4-dioxane in rats". Inhalation toxicology 20 (10): 961–71. doi:10.1080/08958370802105397. PMID 18668411.
- ↑ Kasai, T; Kano, H; Umeda, Y; Sasaki, T; Ikawa, N; Nishizawa, T; Nagano, K; Arito, H; Nagashima, H; Fukushima, S (2009). "Two-year inhalation study of carcinogenicity and chronic toxicity of 1,4-dioxane in male rats". Inhalation toxicology 21 (11): 889–97. doi:10.1080/08958370802629610. PMID 19681729.
- ↑ Kinne, Matthias; Poraj-Kobielska, Marzena; Ralph, Sally A.; Ullrich, René; Hofrichter, Martin; Hammel, Kenneth E. (2009). "Oxidative cleavage of diverse ethers by an extracellular fungal peroxygenase". The Journal of Biological Chemistry 284 (43): 29343–9. doi:10.1074/jbc.M109.040857. PMC 2785565. PMID 19713216.
- ↑ Tenth Report on Carcinogens. U.S. Department of Health and Human Services, Public Health Service, National Toxicology Program, December 2002.
- ↑ "Chemical Encyclopedia: 1,4-dioxane". Healthy Child Healthy World. Archived from the original on 29 November 2009. Retrieved 14 December 2009.
- ↑ "Watchdog issues inspection results on Johnson & Johnson". China Daily (Xinhua). 21 March 2009. Retrieved 14 May 2010.
- ↑ "Carcinogenic 1,4-Dioxane Found in Leading 'Organic' Brand Personal Care Products" (Press release). Organic Consumers Association. 14 March 2008. Archived from the original on 24 September 2010. Retrieved 6 October 2010.
- 1 2 3 Black, RE; Hurley, FJ; Havery, DC (2001). "Occurrence of 1,4-dioxane in cosmetic raw materials and finished cosmetic products". Journal of AOAC International 84 (3): 666–70. PMID 11417628.
- ↑ FDA/CFSAN--Cosmetics Handbook Part 3: Cosmetic Product-Related Regulatory Requirements and Health Hazard Issues. Prohibited Ingredients and other Hazardous Substances: 9. Dioxane