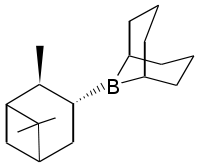
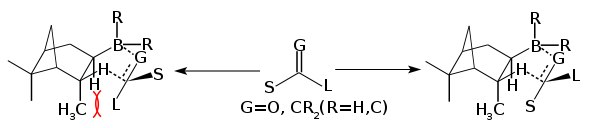Diisopinocampheylborane
2BH.png) | |
| Names | |
|---|---|
| IUPAC name
Bis{(1S,2S,3S,5R)-2,6,6-trimethylbicyclo[3.1.1]heptan-3-yl} borane | |
| Other names
(+)-Di-3-pinanylborane; Diisopinocampheylborane; Ipc2BH | |
| Identifiers | |
| 21947-87-5 | |
| Abbreviations | Ipc2BH |
| ChemSpider | 10618744 |
| Jmol interactive 3D | Image |
| |
| |
| Properties | |
| C20H35B | |
| Molar mass | 286.31 g·mol−1 |
| Appearance | Colorless solid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Diisopinocampheylborane is an organoborane that is useful for asymmetric synthesis. This colourless solid is the precursor to a range of related reagents. The compound was reported in 1961 by Zweifel and Brown in a pioneering demonstration of asymmetric synthesis using boranes. The reagent is mainly used for the synthesis of chiral secondary alcohols.
Preparation
Diisopinocampheylborane was originally prepared by hydroboration of excess α-pinene with borane,[1] but it is now more commonly generated from borane-methyl sulfide (BMS).[2]
Structure, general properties, reactions
The compound can be isolated as a solid, but it is quite sensitive to water and air and is thus often generated in situ and used as a solution. Oxidation of diisopinocampheylborane yields isopincampheol.[3] It adds to alkenes and alkynes to form the corresponding alkyl- and vinyldiisopinocampheylboranes, where G is CHR and R is Ipc and H:
Diisopinocampheylborane is monomeric, in contrast to diborane and many of its less bulky derivatives that contain B-H bonds. Because of the large size of the α-pinenyl groups, diisopinocampheylborane does not hydroborate hindered alkenes but when it does add, it does so with high enantioselectivity, even for small L's. 2-Butene, 2-pentene, 3-hexene are converted with Diisopinocampheylborane to the respective chiral alcohols in high ee's. Norbornene under the same conditions gave an 83% ee, due to the additional steric effects involved. Hetrocycles (dihydrofuran, dihydrothiophene, dihydropyrrole, tetrahydropyran) give the alcohol in ≥99% ee; the high ee's reflect their constrained conformations.[4] In a highly stereoselective reaction, allyldiisopinocampheylboranes converts aldehydes to the homologated alcohols, rapidly even at -100 °C.[5]
The alkyldiisopinocampheylboranes, which result from the addition to alkenes, usefully react with a range of different reagents. Hydroxylamine-O-sulfonic acid provides 3-pinanamine.[6] Hydroboration of 2-butene with diisopinocampheylborane followed by oxidation gives R-(−)-2-butanol in 87% ee.[7][7]
- (isopinocampheyl)2BH + RCH=CHR → (isopinocampheyl)2BCH(R)CH2R
The resulting triorganoborane is oxidized with hydrogen peroxide]] to give the chiral alcohol HOCH(R)CH2R. Also useful is the reaction of diisopinocampheylborane with an aldehyde (RCHO) to give the chiral boronic ester, (isopinocampheyl)2BOCH2(R), which can be further used is a number of reactions e.g. Suzuki reaction.[7]
Related campheylboranes

Treatment of diisopinocampheylborane with TMEDA give the crystalline adduct of monoisopinocampheylborane. This adduct reacts with boron trifluoride to liberate the monoisopinocampheylborane (IpcBH2) in 100% ee.[8] Monoisopinocampheylborane reacts with a variety of alkenes.[7] Two other reagents have been developed for the hydroboration of ketones:
In the above mechanism where G=O and R is Ipc and Cl or 9-Borabicyclononane. Diisopinocampheylchloroborane (Ipc2BCl) is produced by treating diisopinocampheylborane with hydrogen chloride. The chloride is reported to be more stable that the trialkyl boranes,[7] it works well with aryl alkyl ketones and tert-butyl aklyl ketones. Diisopinocampheylchloroborane is often complementary with diisopinocampheylborane, where one provides the R enantiomer and the other the S, the enantioselectivity is typically very high.[9][10]
Alpine-borane is produced by hydroborating α-pinene with 9-borabicyclononane.[7] Both of these reagents can be improved upon by using 2-ethylapopinene in place of α-pinene, 2-ethylapopinene has an ethyl group in place of the methyl in α-pinene. The additional steric bulk improves the stereoselectivity of the reduction.
Diisopinocampheylborane reacts with methanol to give diisopinocampheylmethoxyborane, which in turn reacts with an allyl or crotyl Grignard reagent to give B-allyldiisopinocampheylborane. This can then undergo an asymmertric allylboration to give a chiral homologated alcohol, which is a useful building block in a chiral synthesis.
References
- ↑ Brown, Herbert C.; Yoon, N. M. (1977). Isr. J. Chem. 15: 12–16. Missing or empty
|title=(help) - ↑ Brown, Herbert C.; Bakthan Singaram (1984). "Improved procedures for the synthesis of diisopinocampheylborane of high optical purity". J. Org. Chem. 49 (5): 945–947. doi:10.1021/jo00179a041.
- ↑ C. F. Lane, J. J. Daniels (1972). "(−)-Isopincampheol". Org. Synth. 52: 59.; Coll. Vol. 6, p. 719
- ↑ Brown, Herbert C.; Veeraraghavan Ramachandran (1991). "The boron approach to asymmertric synthesis". Pure & Appl. Chem. 63 (3): 307–316. doi:10.1351/pac199163030307.
- ↑ Raj K. Dhar, Kanth V. B. Josyula, Robert Todd “Diisopinocampheylborane” in Encyclopedia of Reagents for Organic Synthesis, 2006, John Wiley & Sons, New York. doi:10.1002/047084289X.rd248.pub2. Article Online Posting Date: September 15, 2006
- ↑ Michael W. Rathke, Alan A. Millard. (1978). "Boranes in functionalization of olefins to amines: 3-Pinanamine". Org. Synth. 58: 32.; Coll. Vol. 6, p. 943
- 1 2 3 4 5 6 Brown, Herbert C.; George Zweifel (1961). "Hydroboration as a convenient procedure for the asymmetric synthesis of alcohols of high optical purity". J. Am. Chem. Soc. 83 (2): 486–487. doi:10.1021/ja01463a055.
- ↑ Brown, Herbert C.; John R. Schwier; Bakthan Singaram (1978). "Simple synthesis of monoisopinocampheylborane of high optical purity". J. Org. Chem. 43 (32): 4395–4397. doi:10.1021/jo00416a042.
- ↑ Ramachandran, P.Veeraraghavan; Chen, Guang-Ming; Brown, Herbert C. (1996). "Efficient general asymmetric syntheses of 3-substituted 1(3H)-isobenzofuranones in very high enantiomeric excess". Tetrahedron Letters 37 (13): 2205–2208. doi:10.1016/0040-4039(96)00260-2. ISSN 0040-4039.
- ↑ Ramachandran, V. P.; S. Pitre; Herbert C. Brown (2002). "Selective Reductions. 59. Effective Intramolecular Asymmetric Reductions of α-,β and γ-Keto Acids with Diisopinocampheylborane and Intermolecular Asymmetric Reductions of the Corresponding Esters with B-Chlorodiisopinocampheylborane". J. Org. Chem. 67 (15): 5315–5319. doi:10.1021/jo025594y.