Difluoride
| Order and disorder in difluorides | |
_-_cropped.png) |
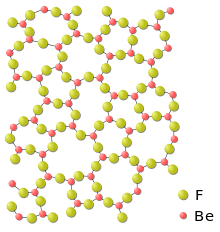 |
| The fluorite structure | Beryllium fluoride glass |
Difluorides are chemical compounds with two fluorine atoms per molecule (or per formula unit).
Metal difluorides are all ionic. Despite being highly ionic, the alkali earth metal difluorides generally have extremely high lattice stability and are thus insoluble in water. One exception is beryllium difluoride. In addition, many transition metal difluorides are water-soluble.
Calcium difluoride is a notable compound. In the form of the mineral fluorite it is the major source of commercial fluorine. It also has an epynomic crystal structure, which is an end member of the spectrum starting from bixbyite and progressing through pyrochlore.
Solubility-related constants of alkaline earth metal fluorides Metal M2+ HE[1] F− HE[2] "MF2" unit
HEMF2 lattice
energies (−kJ/mol)[3]Solubility
(mol/L)[4]Be 2,455 458 3,371 3,526 soluble Mg 1,922 458 2,838 2,978 0.0012 Ca 1,577 458 2,493 2,651 0.0002 Sr 1,415 458 2,331 2,513 0.0008 Ba 1,361 458 2,277 2,373 0.006
List of the difluorides
Examples of the difluorides include:
Metal difluorides
Compounds of the form MF2:
- Beryllium difluoride
- Cadmium difluoride
- Calcium difluoride
- Cobalt difluoride
- Lead difluoride
- Mercury difluoride
- Nickel difluoride
- Palladium difluoride
- Silver difluoride
- Strontium difluoride
- Zinc difluoride
Bifluorides
The bifluorides contain the two fluorine atoms in a covalently bound HF2− polyatomic ion rather than as F− anions.
Nonmetal difluorides
- Krypton difluoride
- Dinitrogen difluoride
- Oxygen difluoride
- Dioxygen difluoride
- Selenoyl difluoride
- Sulfur difluoride
- Disulfur difluoride
- Thionyl difluoride
- Radon difluoride
- Xenon difluoride
Organic difluorides
- Ethanedioyl difluoride
- Ethylidene difluoride
- Carbonyl difluoride
- Carbon dibromide difluoride (dibromodifluoromethane)
- Carbon dichloride difluoride (dichlorodifluormethane)
- Methyl difluoride
- Methylphosphonyl difluoride
- Polyvinylidene difluoride
References
- ↑ Wiberg, Wiberg & Holleman 2001, pp. XXXVI–XXXVII.
- ↑ Wiberg, Wiberg & Holleman 2001, p. XXXVI.
- ↑ Lide 2004, p. 12-23.
- ↑ Wiberg, Wiberg & Holleman 2001, p. 1073.
Bibliography
- Greenwood, N. N.; Earnshaw, A. (1998). Chemistry of the Elements (second edition). Butterworth Heinemann. ISBN 0-7506-3365-4.
- Lide, David R. (2004). Handbook of chemistry and physics (84th ed.). CRC Press. ISBN 0-8493-0566-7.