Diethylamine
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1-ethylaminoethane | |
| Other names
Diethamine; N,N-Diethylamine; Diethylamine | |
| Identifiers | |
| 109-89-7 | |
| 605268 | |
| ChEBI | CHEBI:85259 |
| ChEMBL | ChEMBL1189 |
| ChemSpider | 7730 |
| EC Number | 203-716-3 |
| Jmol interactive 3D | Image |
| MeSH | diethylamine |
| PubChem | 8021 |
| RTECS number | HZ8750000 |
| UNII | B035PIS86W |
| UN number | 1154 |
| |
| |
| Properties | |
| C4H11N | |
| Molar mass | 73.14 g·mol−1 |
| Appearance | Colourless liquid |
| Odor | fishy, ammoniacal |
| Density | 0.7074 g mL−1 |
| Melting point | −49.80 °C; −57.64 °F; 223.35 K |
| Boiling point | 54.8 to 56.4 °C; 130.5 to 133.4 °F; 327.9 to 329.5 K |
| Miscible | |
| log P | 0.657 |
| Vapor pressure | 24.2–97.5 kPa |
| Henry's law constant (kH) |
150 μmol Pa−1 kg−1 |
| Refractive index (nD) |
1.385 |
| Thermochemistry | |
| 178.1 J K−1 mol−1 | |
| Std enthalpy of formation (ΔfH |
−131 kJ mol−1 |
| Std enthalpy of combustion (ΔcH |
−3.035 MJ mol−1 |
| Hazards | |
| Safety data sheet | hazard.com |
| GHS pictograms |  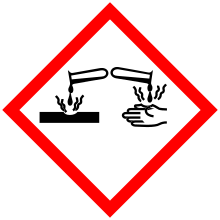  |
| GHS signal word | DANGER |
| H225, H302, H312, H314, H332 | |
| P210, P280, P305+351+338, P310 | |
| EU classification (DSD) |
|
| R-phrases | R11, R20/21/22, R35 |
| S-phrases | (S1/2), S3, S16, S26, S29, S36/37/39 |
| NFPA 704 | |
| Flash point | −23 °C (−9 °F; 250 K) |
| 312 °C (594 °F; 585 K) | |
| Explosive limits | 1.8–10.1% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (Median dose) |
540 mg/kg (rat, oral) 500 mg/kg (mouse, oral)[1] |
| LC50 (Median concentration) |
4000 ppm (rat, 4 hr)[1] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 25 ppm (75 mg/m3)[2] |
| REL (Recommended) |
TWA 10 ppm (30 mg/m3) ST 25 ppm (75 mg/m3)[2] |
| IDLH (Immediate danger |
200 ppm[2] |
| Related compounds | |
| Related amines |
|
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diethylamine is a secondary amine with the molecular structure CH3CH2NHCH2CH3 (also written as C4H11N). It is a flammable, weakly alkaline liquid. It is miscible with water and ethanol. It is a colorless liquid which often appears brown due to impurities. It is volatile and has a strong unpleasant odor.
Diethylamine is manufactured from ethanol and ammonia and is obtained together with ethylamine and triethylamine. It is used as a corrosion inhibitor and in the production of rubber, resins, dyes and pharmaceuticals.
Diethylamine is a corrosive chemical and contact with skin may cause irritation or burns.
Diethylamine can be used to produce LSD and is strictly watched by the DEA.
References
- 1 2 "Diethylamine". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- 1 2 3 "NIOSH Pocket Guide to Chemical Hazards #0209". National Institute for Occupational Safety and Health (NIOSH).
- Merck Index, 12th Edition, 3160.
External links
This article is issued from Wikipedia - version of the Sunday, November 01, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
