Diels–Reese reaction
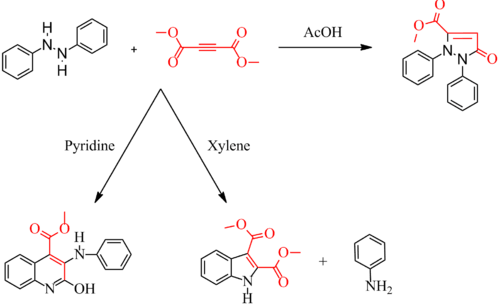
The Diels–Reese Reaction was first reported in 1934 by O. Diels and J. Reese.[1][2] An extension to the reaction was made by William Hearon.[3] The reaction is believed to proceed through an unknown intermediate or adduct as proposed by Hearon. By changing the solvent the resulting product will change. With acetic acid as solvent (acidic pH), the reaction yields the diphenylpyrazolone. With xylene as solvent (neutral pH), the reaction gives the indole, and with pyridine (basic pH), the reaction afforded the carbomethoxyquinoline which can be degraded to the dihydroquinoline.
References
This article is issued from Wikipedia - version of the Friday, February 12, 2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.