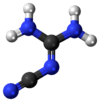
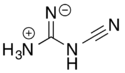
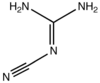2-Cyanoguanidine
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2-Cyanoguanidine | |||
| Other names
Cyanoguanidine, dicyanodiamide, N-cyanoguanidine, 1-cyanoguanidine, Guanidine-1-carbonitrile, dicyandiamin, Didin, DCD, Dicy | |||
| Identifiers | |||
| 461-58-5 | |||
| ChemSpider | 9611 | ||
| EC Number | 207-312-8 | ||
| Jmol interactive 3D | Image | ||
| PubChem | 10005 | ||
| RTECS number | ME9950000 | ||
| |||
| |||
| Properties | |||
| C2H4N4 | |||
| Molar mass | 84.08 g/mol | ||
| Appearance | White crystals | ||
| Density | 1.400 g/cm3 | ||
| Melting point | 209.5 °C (409.1 °F; 482.6 K) | ||
| Boiling point | 252 °C (486 °F; 525 K) | ||
| 41.3 g/l | |||
| log P | -0.52 | ||
| Henry's law constant (kH) |
2.25·10−10 atm.m3/mol | ||
| Hazards | |||
| Main hazards | harmful (Xn) | ||
| R-phrases | R20/21/22 | ||
| S-phrases | S24/25 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
2-Cyanoguanidine is an nitrile derived from guanidine. It is a dimer of cyanamide, from which it can be prepared. 2-Cyanoguanidine is a colourless solid that is soluble in water, acetone, and alcohol, but not nonpolar organic solvents.[1]
Production and use
2-Cyanoguanidine is produced by treating cyanamide with base. It is produced in soil by decomposition of cyanamide. A variety of useful compounds are produced from 2-cyanoguanidine, guanidines and melamine. It is also used as a slow fertilizer. Formerly, it was used as a fuel in some explosives. It is used in the adhesive industry as a curing agent for epoxies.[1]
Chemistry
There are two tautomeric forms, differing in the protonation and bonding of the nitrogen to which the nitrile group is attached.
2-Cyanoguanidine can also exist in a zwitterionic form via a formal acid–base reaction among the nitrogens.
Loss of ammonia (NH3) from the zwitterionic form, followed by deprotonation of the remaining central nitrogen atom, gives the dicyanamide anion, [N(CN)2]−.
Controversy
In September 2012, traces of dicyandiamide were found in milk produced by New Zealand company Fonterra.[2] Fonterra, Federated Farmers, and the Government moved quickly to reassure the public and overseas buyers there was no risk to health.[3] Fonterra has received praise for its handling of the DCD issue.[4] The levels were very low and attempts were made to prevent the test results from being reported in the media.[5]
References
- 1 2 Thomas Güuthner; Bernd Mertschenk (2006). "Cyanamides". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a08_139.pub2.
- ↑ "Government downplays DCD risk". 3 News NZ. January 27, 2013.
- ↑ Astley, Mark (January 28, 2013). "Government defends Fonterra's safety". 3 News NZ.
- ↑ "DCD concerns: NZ handling of crisis was ‘textbook’ - Rabobank". Dairy Reporter. February 21, 2013.
- ↑ Watkins, Tracy (14 April 2013). "Secrecy Over DCD In Milk Scare Revealed". Sunday Star-Times. Retrieved 13 April 2013.