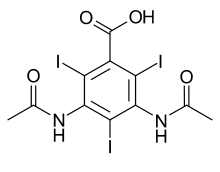
Diatrizoic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3,5-bis(acetylamino)-2,4,6-triiodobenzoic acid | |
| Identifiers | |
| 737-31-5 | |
| ChEBI | CHEBI:53691 |
| ChEMBL | ChEMBL1201220 |
| ChemSpider | 2055 |
| DrugBank | DB00271 |
| Jmol interactive 3D | Image Image |
| KEGG | D02240 |
| PubChem | 2140 |
| |
| |
| Properties | |
| C11H9I3N2O4 | |
| Molar mass | 613.9 g/mol |
| Pharmacology | |
| ATC code | V08 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Diatrizoic acid (or its anionic form, diatrizoate), also known as amidotrizoic acid, or 3,5-diacetamido-2,4,6-triiodobenzoic acid, is a radiocontrast agent containing iodine. Trade names include Hypaque, Gastrografin and Urografin, the latter being a combination of the sodium and meglumine salts.
Diatrizoate is considered a high-osmolality contrast agent. Its osmolality ranges from approximately 1500 mOsm/kg (50% solution)[1] to over 2000 mOsm/kg (76% solution).[2]
Indications
Diatrizoic acid may be used as an alternative to barium sulfate for medical imaging of the gastrointestinal tract, such as upper gastrointestinal series and small bowel series. It is indicated for use in patients who are allergic to barium, or in cases where the barium might leak into the abdominal cavity. It does not coat the stomach/bowel lining as well as barium, so it is not used commonly for this purpose.
Under the trade name Urografin it is used for intravenous pyelography.
It is also used to kill Ascaris lumbricoides (roundworms).[3][4]
Administration
- It is given intravenously (under brand name Hypaque, GE Healthcare) to enhance contrast in computed tomography, to image the kidneys and related structures, and to image blood vessels.
- It is given orally or by enema (Gastrografin, Gastrovist, Gastrovision, MD-Gastroview) to image the gastrointestinal tract.
- It is given by Foley catheter (Cystografin) to image the urinary tract.
Contraindications
A history of sensitivity to iodine is not a contraindication to using diatrizoate, although it suggests caution in use of the agent. In this case, a regimen of oral or intravenous corticosteroids may be given as prophylaxis, or an alternative such as barium sulfate may be preferable.
Gastrografin is contraindicated to use along with certain medications that can cause lactic acidosis, such as metformin. Concurrent use may lead to renal failure and lactic acidosis, and a clinician may need to space the agents apart over a number of days to prevent an interaction.[5]
Gastrografin is a hypertonic solution, and therefore it should be avoided in imaging studies of the upper gastrointestinal tract in patients who are at risk of aspiration, as it will cause prompt pulmonary edema if accidentally introduced into the tracheobronchial tree.
Urografin is not to be used for myelography, ventriculography or cisternography, since it is likely to provoke neurotoxic symptoms in these examinations. [6]
References
- ↑ Amersham Health (April 2006). "Hypaque sodium (Diatrizoate Sodium) injection, solution. Product label". DailyMed. U.S. National Library of Medicine. Retrieved 2007-03-29.
- ↑ Amersham Health (April 2006). "Hypaque (Diatrizoate Meglumine and Diatrizoate Sodium) injection, solution. Product label". DailyMed. U.S. National Library of Medicine. Retrieved 2007-03-29.
- ↑ Sood. Surgical Diseases in Tropical Countries. Jaypee Brothers Publishers. pp. 72–. ISBN 978-81-7179-444-7. Retrieved 11 August 2010.
- ↑ Moshe Schein; Paul Rogers; Ahmad Assalia (2010). Schein's Common Sense Emergency Abdominal Surgery. Springer. pp. 391–. ISBN 978-3-540-74820-5. Retrieved 11 August 2010.
- ↑ Micromedex Healthcare Series (November 2010). "DIATRIZOATE MEGLUMINE/DIATRIZOATE SODIUM". DRUGDEX. Thomson Reuters (Healthcare) Inc. Retrieved 2010-11-10.
- ↑ Bayer New Zealand Limited (September 2007). "UROGRAFIN Urografin Corporate Core Text" (PDF). Retrieved 2012-04-02.
| ||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||