Diabetes management
The term diabetes mellitus includes several different metabolic disorders that all, if left untreated, result in abnormally high concentration of a sugar called glucose in the blood. Diabetes mellitus type 1 results when the pancreas no longer produces significant amounts of the hormone insulin, usually owing to the autoimmune destruction of the insulin-producing beta cells of the pancreas. Diabetes mellitus type 2, in contrast, is now thought to result from autoimmune attacks on the pancreas and/or insulin resistance. The pancreas of a person with type 2 diabetes may be producing normal or even abnormally large amounts of insulin. Other forms of diabetes mellitus, such as the various forms of maturity onset diabetes of the young, may represent some combination of insufficient insulin production and insulin resistance. Some degree of insulin resistance may also be present in a person with type 1 diabetes.
The main goal of diabetes management is, as far as possible, to restore carbohydrate metabolism to a normal state. To achieve this goal, individuals with an absolute deficiency of insulin require insulin replacement therapy, which is given through injections or an insulin pump. Insulin resistance, in contrast, can be corrected by dietary modifications and exercise. Other goals of diabetes management are to prevent or treat the many complications that can result from the disease itself and from its treatment.
Overview
Goals
The treatment goals are related to effective control of blood glucose, blood pressure and lipids, to minimize the risk of long-term consequences associated with diabetes. They are suggested in clinical practice guidelines released by various national and international diabetes agencies.
The targets are:
- HbA1c of 6%[1] to 7.0%[2]
- Preprandial blood glucose: 3.9 to 7.2 mmol/L (70 to 130 mg/dl)[3]
- 2-hour postprandial blood glucose: <10 mmol/L (<180 mg/dl)[3]
Goals should be individualized based on:[3]
- Duration of diabetes
- Age/life expectancy
- Comorbidity
- Known Cardiovascular disease or advanced microvascular disease
- Hypoglycemia awareness
In older patients, clinical practice guidelines by the American Geriatrics Society states "for frail older adults, persons with life expectancy of less than 5 years, and others in whom the risks of intensive glycemic control appear to outweigh the benefits, a less stringent target such as HbA1c of 8% is appropriate".[4]
Issues
The primary issue requiring management is that of the glucose cycle. In this, glucose in the bloodstream is made available to cells in the body; a process dependent upon the twin cycles of glucose entering the bloodstream, and insulin allowing appropriate uptake into the body cells. Both aspects can require management.
Complexities
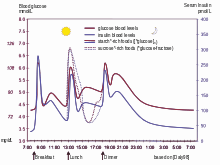
The main complexities stem from the nature of the feedback loop of the glucose cycle, which is sought to be regulated:
- The glucose cycle is a system which is affected by two factors: entry of glucose into the bloodstream and also blood levels of insulin to control its transport out of the bloodstream
- As a system, it is sensitive to diet and exercise
- It is affected by the need for user anticipation due to the complicating effects of time delays between any activity and the respective impact on the glucose system
- Management is highly intrusive, and compliance is an issue, since it relies upon user lifestyle change and often upon regular sampling and measuring of blood glucose levels, multiple times a day in many cases
- It changes as people grow and develop
- It is highly individual
As diabetes is a prime risk factor for cardiovascular disease, controlling other risk factors which may give rise to secondary conditions, as well as the diabetes itself, is one of the facets of diabetes management. Checking cholesterol, LDL, HDL and triglyceride levels may indicate hyperlipoproteinemia, which may warrant treatment with hypolipidemic drugs. Checking the blood pressure and keeping it within strict limits (using diet and antihypertensive treatment) protects against the retinal, renal and cardiovascular complications of diabetes. Regular follow-up by a podiatrist or other foot health specialists is encouraged to prevent the development of diabetic foot. Annual eye exams are suggested to monitor for progression of diabetic retinopathy.
Early advancements
Late in the 19th century, sugar in the urine (glycosuria) was associated with diabetes. Various doctors studied the connection. Frederick Madison Allen studied diabetes in 1909-12, then published a large volume, Studies Concerning Glycosuria and Diabetes, (Boston, 1913). He invented a fasting treatment for diabetes called the Allen treatment for diabetes. His diet was an early attempt at managing diabetes.
Modern approaches
Modern approaches to diabetes primarily rely upon dietary and lifestyle management, often combined with regular ongoing blood glucose level monitoring.
Diet management allows control and awareness of the types of nutrients entering the digestive system, and hence allows indirectly, significant control over changes in blood glucose levels. Blood glucose monitoring allows verification of these, and closer control, especially important since some symptoms of diabetes are not easy for the patient to notice without actual measurement.
Other approaches include exercise and other lifestyle changes which impact the glucose cycle.
In addition, a strong partnership between the patient and the primary healthcare provider – general practitioner or internist – is an essential tool in the successful management of diabetes. Often the primary care doctor makes the initial diagnosis of diabetes and provides the basic tools to get the patient started on a management program. Regular appointments with the primary care physician and a certified diabetes educator are some of the best things a patient can do in the early weeks after a diagnosis of diabetes. Upon the diagnosis of diabetes, the primary care physician, specialist, or endocrinologist will conduct a full physical and medical examination. A thorough assessment covers topics such as:
- Medical history
- Family history of diabetes, cardiovascular disease, and stroke
- Eating and exercise habits; growth and development in children and adolescents
- Diabetes education history
- Review of previous treatment regimen and response to therapy (HbA1c records)
- A list of current medications, including:
- Prescription medications
- Over-the-counter medications
- Vitamin, mineral or herbal supplements
- Smoking history, including encouragement of smoking cessation (if applicable)
- Current treatment plan, including medications, medication compliance and barriers, meal plan, physical activity patterns, readiness for behavior change
- Results of glucose monitoring and patient's use of data
- Diabetic ketoacidosis frequency, severity, and cause
- Hypoglycemic episodes and awareness
- History of diabetes-related complications
- Microvascular: retinopathy, nephropathy, neuropathy
- Macrovascular: coronary artery disease, cerebrovascular disease, peripheral artery disease
- Urination abnormalities, which can indicate kidney disease
- Signs of complications with pregnancy or trying to get pregnant for women patients
- Physical examination
- Height, weight, body mass index
- Blood pressure measurements
- Funduscopy
- Thyroid palpation
- Skin examination at insulin injection sites
- Comprehensive examination of hands, fingers, feet, and toes for circulatory abnormalities
- Additional laboratory evaluation
- If results not available within past 3 months
- If results not available within past year
- Fasting lipid profile: including total, LDL, HDL cholesterol and triglycerides
- Liver function test
- Test for urine albumin excretion with spot urine albumin/creatinine ratio
- Thyroid-stimulating hormone in type 1 diabetes, dyslipidemia, or women over 50 years old
- Referrals
- Eye care profession for annual dilated fundus examination
- Family planning for women of reproductive age
- Registered dietitian for medical nutrition therapy
- Diabetes self-management education (DSME)
- Dentist for comprehensive periodontal exam
- Mental health professional if needed
Diabetes can be very complicated, and the physician needs to have as much information as possible to help the patient establish an effective management plan. Physicians may often experience data overload resulting from hundreds of blood-glucose readings, insulin dosages and other health factors occurring between regular office visits which must be deciphered during a relatively brief visit with the patient to determine patterns and establish or modify a treatment plan.[5]
The physician can also make referrals to a wide variety of professionals for additional health care support. In the UK a patient training course is available for newly diagnosed diabetics (see DESMOND). In big cities, there may be diabetes centers where several specialists, such as diabetes educators and dietitians, work together as a team. In smaller towns, the health care team may come together a little differently depending on the types of practitioners in the area. By working together, doctors and patients can optimize the healthcare team to successfully manage diabetes over the long term.
The 10 countries with the largest populations of diabetic patients are China, India, the U.S., Brazil, Russia, Mexico, Indonesia, Germany, Egypt and Japan.[6]
Blood sugar level
Blood sugar level is measured by means of a glucose meter, with the result either in mg/dL (milligrams per deciliter in the USA) or mmol/L (millimoles per litre in Canada and Europe) of blood. The average normal person has an average fasting glucose level of 4.5 mmol/L (81 mg/dL), with a lows of down to 2.5 and up to 5.4 mmol/L (65 to 98 mg/dL).[7]
Optimal management of diabetes involves patients measuring and recording their own blood glucose levels. By keeping a diary of their own blood glucose measurements and noting the effect of food and exercise, patients can modify their lifestyle to better control their diabetes. For patients on insulin, patient involvement is important in achieving effective dosing and timing.
Some edible mushrooms are noted for the ability to lower blood sugar levels including Reishi,[8][9] Maitake[10][11][12][13][14][15] Agaricus blazei[16][17][18][19] as well as some others.
Hypo and hyperglycemia
Levels which are significantly above or below this range are problematic and can in some cases be dangerous. A level of <3.8 mmol/L (<70 mg/dL) is usually described as a hypoglycemic attack (low blood sugar). Most diabetics know when they are going to "go hypo" and usually are able to eat some food or drink something sweet to raise levels. A patient who is hyperglycemic (high glucose) can also become temporarily hypoglycemic, under certain conditions (e.g. not eating regularly, or after strenuous exercise, followed by fatigue). Intensive efforts to achieve blood sugar levels close to normal have been shown to triple the risk of the most severe form of hypoglycemia, in which the patient requires assistance from by-standers in order to treat the episode.[20] In the United States, there were annually 48,500 hospitalizations for diabetic hypoglycemia and 13,100 for diabetic hypoglycemia resulting in coma in the period 1989 to 1991, before intensive blood sugar control was as widely recommended as today.[21] One study found that hospital admissions for diabetic hypoglycemia increased by 50% from 1990-1993 to 1997-2000, as strict blood sugar control efforts became more common.[22] Among intensively controlled type 1 diabetics, 55% of episodes of severe hypoglycemia occur during sleep, and 6% of all deaths in diabetics under the age of 40 are from nocturnal hypoglycemia in the so-called 'dead-in-bed syndrome,' while National Institute of Health statistics show that 2% to 4% of all deaths in diabetics are from hypoglycemia.[23] In children and adolescents following intensive blood sugar control, 21% of hypoglycemic episodes occurred without explanation.[24] In addition to the deaths caused by diabetic hypoglycemia, periods of severe low blood sugar can also cause permanent brain damage.[25] Interestingly, although diabetic nerve disease is usually associated with hyperglycemia, hypoglycemia as well can initiate or worsen neuropathy in diabetics intensively struggling to reduce their hyperglycemia.[26]
Levels greater than 13-15 mmol/L (230–270 mg/dL) are considered high, and should be monitored closely to ensure that they reduce rather than continue to remain high. The patient is advised to seek urgent medical attention as soon as possible if blood sugar levels continue to rise after 2-3 tests. High blood sugar levels are known as hyperglycemia, which is not as easy to detect as hypoglycemia and usually happens over a period of days rather than hours or minutes. If left untreated, this can result in diabetic coma and death.

Prolonged and elevated levels of glucose in the blood, which is left unchecked and untreated, will, over time, result in serious diabetic complications in those susceptible and sometimes even death. There is currently no way of testing for susceptibility to complications. Diabetics are therefore recommended to check their blood sugar levels either daily or every few days. There is also diabetes management software available from blood testing manufacturers which can display results and trends over time. Type 1 diabetics normally check more often, due to insulin therapy.
A history of blood sugar level results is especially useful for the diabetic to present to their doctor or physician in the monitoring and control of the disease. Failure to maintain a strict regimen of testing can accelerate symptoms of the condition, and it is therefore imperative that any diabetic patient strictly monitor their glucose levels regularly.
Glycemic control
Glycemic control is a medical term referring to the typical levels of blood sugar (glucose) in a person with diabetes mellitus. Much evidence suggests that many of the long-term complications of diabetes, especially the microvascular complications, result from many years of hyperglycemia (elevated levels of glucose in the blood). Good glycemic control, in the sense of a "target" for treatment, has become an important goal of diabetes care, although recent research suggests that the complications of diabetes may be caused by genetic factors[27] or, in type 1 diabetics, by the continuing effects of the autoimmune disease which first caused the pancreas to lose its insulin-producing ability.[28]
Because blood sugar levels fluctuate throughout the day and glucose records are imperfect indicators of these changes, the percentage of hemoglobin which is glycosylated is used as a proxy measure of long-term glycemic control in research trials and clinical care of people with diabetes. This test, the hemoglobin A1c or glycosylated hemoglobin reflects average glucoses over the preceding 2–3 months. In nondiabetic persons with normal glucose metabolism the glycosylated hemoglobin is usually 4-6% by the most common methods (normal ranges may vary by method).
"Perfect glycemic control" would mean that glucose levels were always normal (70–130 mg/dl, or 3.9-7.2 mmol/L) and indistinguishable from a person without diabetes. In reality, because of the imperfections of treatment measures, even "good glycemic control" describes blood glucose levels that average somewhat higher than normal much of the time. In addition, one survey of type 2 diabetics found that they rated the harm to their quality of life from intensive interventions to control their blood sugar to be just as severe as the harm resulting from intermediate levels of diabetic complications.[29]
Accepted "target levels" of glucose and glycosylated hemoglobin that are considered good control have been lowered over the last 25 years, because of improvements in the tools of diabetes care, because of increasing evidence of the value of glycemic control in avoiding complications, and by the expectations of both patients and physicians. What is considered "good control" also varies by age and susceptibility of the patient to hypoglycemia.
In the 1990s the American Diabetes Association conducted a publicity campaign to persuade patients and physicians to strive for average glucose and hemoglobin A1c values below 200 mg/dl (11 mmol/l) and 8%. Currently many patients and physicians attempt to do better than that.
Poor glycemic control refers to persistently elevated blood glucose and glycosylated hemoglobin levels, which may range from 200–500 mg/dl (11-28 mmol/L) and 9-15% or higher over months and years before severe complications occur. Meta-analysis of large studies done on the effects of tight vs. conventional, or more relaxed, glycemic control in type 2 diabetics have failed to demonstrate a difference in all-cause cardiovascular death, non-fatal stroke, or limb amputation, but decreased the risk of nonfatal heart attack by 15%. Additionally, tight glucose control decreased the risk of progression of retinopathy and nephropathy, and decreased the incidence peripheral neuropathy, but increased the risk of hypoglycemia 2.4 times.[30]
Monitoring

Relying on their own perceptions of symptoms of hyperglycemia or hypoglycemia is usually unsatisfactory as mild to moderate hyperglycemia causes no obvious symptoms in nearly all patients. Other considerations include the fact that, while food takes several hours to be digested and absorbed, insulin administration can have glucose lowering effects for as little as 2 hours or 24 hours or more (depending on the nature of the insulin preparation used and individual patient reaction). In addition, the onset and duration of the effects of oral hypoglycemic agents vary from type to type and from patient to patient.
Personal (home) glucose monitoring
Control and outcomes of both types 1 and 2 diabetes may be improved by patients using home glucose meters to regularly measure their glucose levels. Glucose monitoring is both expensive (largely due to the cost of the consumable test strips) and requires significant commitment on the part of the patient. The effort and expense may be worthwhile for patients when they use the values to sensibly adjust food, exercise, and oral medications or insulin. These adjustments are generally made by the patients themselves following training by a clinician.
Regular blood testing, especially in type 1 diabetics, is helpful to keep adequate control of glucose levels and to reduce the chance of long term side effects of the disease. There are many (at least 20+) different types of blood monitoring devices available on the market today; not every meter suits all patients and it is a specific matter of choice for the patient, in consultation with a physician or other experienced professional, to find a meter that they personally find comfortable to use. The principle of the devices is virtually the same: a small blood sample is collected and measured. In one type of meter, the electrochemical, a small blood sample is produced by the patient using a lancet (a sterile pointed needle). The blood droplet is usually collected at the bottom of a test strip, while the other end is inserted in the glucose meter. This test strip contains various chemicals so that when the blood is applied, a small electrical charge is created between two contacts. This charge will vary depending on the glucose levels within the blood. In older glucose meters, the drop of blood is placed on top of a strip. A chemical reaction occurs and the strip changes color. The meter then measures the color of the strip optically.
Self-testing is clearly important in type I diabetes where the use of insulin therapy risks episodes of hypoglycaemia and home-testing allows for adjustment of dosage on each administration.[31] However its benefit in type 2 diabetes is more controversial as there is much more variation in severity of type 2 cases.[32] It has been suggested that some type 2 patients might do as well with home urine-testing alone.[33] The best use of home blood-sugar monitoring is being researched.[34]
Benefits of control and reduced hospital admission have been reported.[35] However, patients on oral medication who do not self-adjust their drug dosage will miss many of the benefits of self-testing, and so it is questionable in this group. This is particularly so for patients taking monotherapy with metformin who are not at risk of hypoglycaemia. Regular 6 monthly laboratory testing of HbA1c (glycated haemoglobin) provides some assurance of long-term effective control and allows the adjustment of the patient's routine medication dosages in such cases. High frequency of self-testing in type 2 diabetes has not been shown to be associated with improved control.[36] The argument is made, though, that type 2 patients with poor long term control despite home blood glucose monitoring, either have not had this integrated into their overall management, or are long overdue for tighter control by a switch from oral medication to injected insulin.[37]
Continuous Glucose Monitoring (CGM) CGM technology has been rapidly developing to give people living with diabetes an idea about the speed and direction of their glucose changes. While it still requires calibration from SMBG and is not indicated for use in correction boluses, the accuracy of these monitors are increasing with every innovation.
HbA1c test
A useful test that has usually been done in a laboratory is the measurement of blood HbA1c levels. This is the ratio of glycated hemoglobin in relation to the total hemoglobin. Persistent raised plasma glucose levels cause the proportion of these molecules to go up. This is a test that measures the average amount of diabetic control over a period originally thought to be about 3 months (the average red blood cell lifetime), but more recently thought to be more strongly weighted to the most recent 2 to 4 weeks. In the non-diabetic, the HbA1c level ranges from 4.0-6.0%; patients with diabetes mellitus who manage to keep their HbA1c level below 6.5% are considered to have good glycemic control. The HbA1c test is not appropriate if there has been changes to diet or treatment within shorter time periods than 6 weeks or there is disturbance of red cell aging (e.g. recent bleeding or hemolytic anemia) or a hemoglobinopathy (e.g. sickle cell disease). In such cases the alternative Fructosamine test is used to indicate average control in the preceding 2 to 3 weeks.
Continuous Glucose Monitoring
The first CGM device made available to consumers was the GlucoWatch biographer in 1999. This product is no longer sold. It was a retrospective device rather than live. Several live monitoring devices have subsequently been manufactured which provide ongoing monitoring of glucose levels on an automated basis during the day, for example:
- The Minimed Paradigm REAL-Time by Minimed, is a continuous glucose monitoring system (CGMS) that provides blood glucose measurements to be made every five minutes over a three-day period. The patient can thus adjust an insulin infusion pump immediately and mimic the "feed-back" mechanism of a pancreas. This is no longer sold. The Enlite Sensor is what they currently sell.
- The Dexcom Seven by Dexcom, is another blood glucose monitoring device. like Minimeds Paradigm it provides measurement every 5 minutes. The sensors lasts 7 days (against medtronics 3 day sensor) before they have to be changed. This is no longer sold. The G4 with Share is what they currently sell.
- Freestyle Libre, a Flash Glucose Monitor.
- Abbott FreeStyle Navigator
- Medtronic Guardian
- The US Food and Drug Administration first approved a non-invasive blood glucose monitoring device, the GlucoWatch G2 Biographer. This allows checking blood glucose levels, while puncturing the skin as little as twice a day. Once calibrated with a blood sample, it pulls body fluids from the skin using small electrical currents, taking six readings an hour for as long as thirteen hours. It has not proven to be reliable enough, or convenient enough to be used in lieu of conventional blood monitoring. Other non-invasive methods like radio waves, ultrasound and energy waves are also being tested. The accuracies of these non-invasive devices are at the current stage behind the devices that are inserted or operated into the body. This is no longer sold.
Lifestyle modification
Diet
For Type 1 diabetics there will always be a need for insulin injections throughout their life. However, both Type 1 and Type 2 diabetics can see dramatic effects on their blood sugars through controlling their diet, and some Type 2 diabetics can fully control the disease by dietary modification. As diabetes can lead to many other complications it is critical to maintain blood sugars as close to normal as possible and diet is the leading factor in this level of control.
Recent research shows that the first step in Diabetes management should be for patients to be put on a low carb diet. Patients that are put on a high carb diet find it very difficult to maintain normal blood glucose levels. Patients that are put on a low carb or restricted carbohydrate diet, manage to maintain near normal blood glucose levels and A1cs.[38][39][40][41][42][43][44][45][46]
Medications
Currently, one goal for diabetics is to avoid or minimize chronic diabetic complications, as well as to avoid acute problems of hyperglycemia or hypoglycemia. Adequate control of diabetes leads to lower risk of complications associated with unmonitored diabetes including kidney failure (requiring dialysis or transplant), blindness, heart disease and limb amputation. The most prevalent form of medication is hypoglycemic treatment through either oral hypoglycemics and/or insulin therapy. There is emerging evidence that full-blown diabetes mellitus type 2 can be evaded in those with only mildly impaired glucose tolerance.[47]
Patients with type 1 diabetes mellitus require direct injection of insulin as their bodies cannot produce enough (or even any) insulin. As of 2010, there is no other clinically available form of insulin administration other than injection for patients with type 1: injection can be done by insulin pump, by jet injector, or any of several forms of hypodermic needle. Non-injective methods of insulin administration have been unattainable as the insulin protein breaks down in the digestive tract. There are several insulin application mechanisms under experimental development as of 2004, including a capsule that passes to the liver and delivers insulin into the bloodstream.[48] There have also been proposed vaccines for type I using glutamic acid decarboxylase (GAD), but these are currently not being tested by the pharmaceutical companies that have sublicensed the patents to them.
For type 2 diabetics, diabetic management consists of a combination of diet, exercise, and weight loss, in any achievable combination depending on the patient. Obesity is very common in type 2 diabetes and contributes greatly to insulin resistance. Weight reduction and exercise improve tissue sensitivity to insulin and allow its proper use by target tissues.[49] Patients who have poor diabetic control after lifestyle modifications are typically placed on oral hypoglycemics. Some Type 2 diabetics eventually fail to respond to these and must proceed to insulin therapy. A study conducted in 2008 found that increasingly complex and costly diabetes treatments are being applied to an increasing population with type 2 diabetes. Data from 1994 to 2007 was analyzed and it was found that the mean number of diabetes medications per treated patient increased from 1.14 in 1994 to 1.63 in 2007.[50]
Patient education and compliance with treatment is very important in managing the disease. Improper use of medications and insulin can be very dangerous causing hypo- or hyper-glycemic episodes.
Insulin
Insulin therapy requires close monitoring and a great deal of patient education, as improper administration is quite dangerous. For example, when food intake is reduced, less insulin is required. A previously satisfactory dosing may be too much if less food is consumed causing a hypoglycemic reaction if not intelligently adjusted. Exercise decreases insulin requirements as exercise increases glucose uptake by body cells whose glucose uptake is controlled by insulin, and vice versa. In addition, there are several types of insulin with varying times of onset and duration of action.
Insulin therapy creates risk because of the inability to continuously know a person's blood glucose level and adjust insulin infusion appropriately. New advances in technology have overcome much of this problem. Small, portable insulin infusion pumps are available from several manufacturers. They allow a continuous infusion of small amounts of insulin to be delivered through the skin around the clock, plus the ability to give bolus doses when a person eats or has elevated blood glucose levels. This is very similar to how the pancreas works, but these pumps lack a continuous "feed-back" mechanism. Thus, the user is still at risk of giving too much or too little insulin unless blood glucose measurements are made.
A further danger of insulin treatment is that while diabetic microangiopathy is usually explained as the result of hyperglycemia, studies in rats indicate that the higher than normal level of insulin diabetics inject to control their hyperglycemia may itself promote small blood vessel disease.[26] While there is no clear evidence that controlling hyperglycemia reduces diabetic macrovascular and cardiovascular disease, there are indications that intensive efforts to normalize blood glucose levels may worsen cardiovascular and cause diabetic mortality.[51]
Driving

Studies conducted in the United States[52] and Europe[53] showed that drivers with type 1 diabetes had twice as many collisions as their non-diabetic spouses, demonstrating the increased risk of driving collisions in the type 1 diabetes population. Diabetes can compromise driving safety in several ways. First, long-term complications of diabetes can interfere with the safe operation of a vehicle. For example, diabetic retinopathy (loss of peripheral vision or visual acuity), or peripheral neuropathy (loss of feeling in the feet) can impair a driver’s ability to read street signs, control the speed of the vehicle, apply appropriate pressure to the brakes, etc.
Second, hypoglycemia can affect a person’s thinking process, coordination, and state of consciousness.[54][55] This disruption in brain functioning is called neuroglycopenia. Studies have demonstrated that the effects of neuroglycopenia impair driving ability.[54][56] A study involving people with type 1 diabetes found that individuals reporting two or more hypoglycemia-related driving mishaps differ physiologically and behaviorally from their counterparts who report no such mishaps.[57] For example, during hypoglycemia, drivers who had two or more mishaps reported fewer warning symptoms, their driving was more impaired, and their body released less epinephrine (a hormone that helps raise BG). Additionally, individuals with a history of hypoglycemia-related driving mishaps appear to use sugar at a faster rate[58] and are relatively slower at processing information.[59] These findings indicate that although anyone with type 1 diabetes may be at some risk of experiencing disruptive hypoglycemia while driving, there is a subgroup of type 1 drivers who are more vulnerable to such events.
Given the above research findings, it is recommended that drivers with type 1 diabetes with a history of driving mishaps should never drive when their BG is less than 70 mg/dl (3.9 mmol/l). Instead, these drivers are advised to treat hypoglycemia and delay driving until their BG is above 90 mg/dl (5 mmol/l).[57] Such drivers should also learn as much as possible about what causes their hypoglycemia, and use this information to avoid future hypoglycemia while driving.
Studies funded by the National Institutes of Health (NIH) have demonstrated that face-to-face training programs designed to help individuals with type 1 diabetes better anticipate, detect, and prevent extreme BG can reduce the occurrence of future hypoglycemia-related driving mishaps.[60][61][62] An internet-version of this training has also been shown to have significant beneficial results.[63] Additional NIH funded research to develop internet interventions specifically to help improve driving safety in drivers with type 1 diabetes is currently underway.[64]
Exenatide
The U.S. Food and Drug Administration (FDA) has approved a treatment called Exenatide, based on the saliva of a Gila monster, to control blood sugar in patients with type 2 diabetes.
Other regimens
Artificial Intelligence researcher Dr. Cynthia Marling, of the Ohio University Russ College of Engineering and Technology, in collaboration with the Appalachian Rural Health Institute Diabetes Center, is developing a case based reasoning system to aid in diabetes management. The goal of the project is to provide automated intelligent decision support to diabetes patients and their professional care providers by interpreting the ever increasing quantities of data provided by current diabetes management technology and translating it into better care without time consuming manual effort on the part of an endocrinologist or diabetologist.[65] This type of Artificial Intelligence-based treatment shows some promise with initial testing of a prototype system producing best practice treatment advice which anaylizing physicians deemed to have some degree of benefit over 70% of the time and advice of neutral benefit another nearly 25% of the time.[5]
Use of a "Diabetes Coach" is becoming an increasingly popular way to manage diabetes. A Diabetes Coach is usually a Certified diabetes educator (CDE) who is trained to help people in all aspects of caring for their diabetes. The CDE can advise the patient on diet, medications, proper use of insulin injections and pumps, exercise, and other ways to manage diabetes while living a healthy and active lifestyle. CDEs can be found locally or by contacting a company which provides personalized diabetes care using CDEs. Diabetes Coaches can speak to a patient on a pay-per-call basis or via a monthly plan.
Dental care
High blood glucose in diabetic people is a risk factor for developing gum and teeth problems, especially in post puberty and aging individuals. Diabetic patients have greater chances of developing oral health problems such as tooth decay, salivary gland dysfunction, fungal infections, inflammatory skin disease, periodontal disease or taste impairment and thrush of the mouth.[66] The oral problems in persons suffering from diabetes can be prevented with a good control of the blood sugar levels, regular check-ups and a very good oral hygiene. By maintaining a good oral status, diabetic persons prevent losing their teeth as a result of various periodontal conditions.
Diabetic persons must increase their awareness towards the oral infections as they have a double impact on one's health. Firstly, people with diabetes are more likely to develop periodontal disease which causes increased blood sugar levels, often leading to diabetes complications. Severe periodontal disease can increase blood sugar, contributing to increased periods of time when the body functions with a high blood sugar. This puts diabetics at increased risk for diabetic complications.[67]
The first symptoms of gum and teeth infections in diabetic persons are decreased salivary flow, burning mouth or tongue. Also, patients may experience signs as dry mouth which increases the incidence of decay. Poorly controlled diabetes usually leads to gum problems recession as plaque creates more harmful proteins in the gums.
Tooth decay and cavities are some of the first oral problems that individuals with diabetes are at risk for. Increased blood sugar levels translate into greater sugars and acids that attack the teeth and lead to gum diseases. Gingivitis can also occur as a result of increased blood sugar levels along with an inappropriate oral hygiene. Periodontitis is an oral disease caused by untreated gingivitis and which destroys the soft tissue and bone that support the teeth. This disease may cause the gums to pull away from the teeth which may eventually loosen and fall out. Diabetic people tend to experience more severe periodontitis because diabetes lowers the ability to resist infection[68] and also slows healing. At the same time, an oral infection such as periodontitis can make diabetes more difficult to control because it causes the blood sugar levels to rise.[69]
To prevent further diabetic complications as well as serious oral problems, diabetic persons must keep their blood sugar levels under control and have a proper oral hygiene. A study in the Journal of Periodontology found that poorly controlled type 2 diabetic patients are more likely to develop periodontal disease than well-controlled diabetics are.[67] At the same time, diabetic patients are recommended to have regular checkups with a dental care provider at least once in three to four months. Diabetics who receive good dental care and have good insulin control typically have a better chance at avoiding gum disease to help prevent tooth loss.[70]
Dental care is therefore even more important for diabetic patients than for healthy individuals. Maintaining the teeth and gum healthy is done by taking some preventing measures such as regular appointments at a dentist and a very good oral hygiene. Also, oral health problems can be avoided by closely monitoring the blood sugar levels. Patients who keep better under control their blood sugar levels and diabetes are less likely to develop oral health problems when compared to diabetic patients who control their disease moderately or poorly.
Poor oral hygiene is a great factor to take under consideration when it comes to oral problems and even more in people with diabetes. Diabetic people are advised to brush their teeth at least twice a day, and if possible, after all meals and snacks. However, brushing in the morning and at night is mandatory as well as flossing and using an anti-bacterial mouthwash. Individuals who suffer from diabetes are recommended to use toothpaste that contains fluoride as this has proved to be the most efficient in fighting oral infections and tooth decay. Flossing must be done at least once a day, as well because it is helpful in preventing oral problems by removing the plaque between the teeth, which is not removed when brushing.
Diabetic patients must get professional dental cleanings every six months. In cases when dental surgery is needed, it is necessary to take some special precautions such as adjusting diabetes medication or taking antibiotics to prevent infection. Looking for early signs of gum disease (redness, swelling, bleeding gums) and informing the dentist about them is also helpful in preventing further complications. Quitting smoking is recommended to avoid serious diabetes complications and oral diseases.
Diabetic persons are advised to make morning appointments to the dental care provider as during this time of the day the blood sugar levels tend to be better kept under control. Not least, individuals who suffer from diabetes must make sure both their physician and dental care provider are informed and aware of their condition, medical history and periodontal status.
Medication nonadherence
Because many patients with diabetes have two or more comorbidities, they often require multiple medications. The prevalence of medication nonadherence is high among patients with chronic conditions, such as diabetes, and nonadherence is associated with public health issues and higher health care costs. One reason for nonadherence is the cost of medications. Being able to detect cost-related nonadherence is important for health care professionals, because this can lead to strategies to assist patients with problems paying for their medications. Some of these strategies are use of generic drugs or therapeutic alternatives, substituting a prescription drug with an over-the-counter medication, and pill-splitting. Interventions to improve adherence can achieve reductions in diabetes morbidity and mortality, as well as significant cost savings to the health care system.[71]
Research
Type 1 diabetes
Diabetes type 1 is caused by the destruction of enough beta cells to produce symptoms; these cells, which are found in the Islets of Langerhans in the pancreas, produce and secrete insulin, the single hormone responsible for allowing glucose to enter from the blood into cells (in addition to the hormone amylin, another hormone required for glucose homeostasis). Hence, the phrase "curing diabetes type 1" means "causing a maintenance or restoration of the endogenous ability of the body to produce insulin in response to the level of blood glucose" and cooperative operation with counterregulatory hormones.
This section deals only with approaches for curing the underlying condition of diabetes type 1, by enabling the body to endogenously, in vivo, produce insulin in response to the level of blood glucose. It does not cover other approaches, such as, for instance, closed-loop integrated glucometer/insulin pump products, which could potentially increase the quality-of-life for some who have diabetes type 1, and may by some be termed "artificial pancreas".
Encapsulation approach
A biological approach to the artificial pancreas is to implant bioengineered tissue containing islet cells, which would secrete the amounts of insulin, amylin and glucagon needed in response to sensed glucose.
When islet cells have been transplanted via the Edmonton protocol, insulin production (and glycemic control) was restored, but at the expense of continued immunosuppression drugs. Encapsulation of the islet cells in a protective coating has been developed to block the immune response to transplanted cells, which relieves the burden of immunosuppression and benefits the longevity of the transplant.[72]
Stem cells
Research is being done at several locations in which islet cells are developed from stem cells.
Brazil
Stem cell research has also been suggested as a potential avenue for a cure since it may permit regrowth of Islet cells which are genetically part of the treated individual, thus perhaps eliminating the need for immuno-suppressants.[48] This new method autologous nonmyeloablative hematopoietic stem cell transplantation was developed by a research team composed by Brazilian and American scientists (Dr. Julio Voltarelli, Dr. Carlos Eduardo Couri, Dr Richard Burt, and colleagues) and it was the first study to use stem cell therapy in human diabetes mellitus This was initially tested in mice and in 2007 there was the first publication of stem cell therapy to treat this form of diabetes.[73] Until 2009, there was 23 patients included and followed for a mean period of 29.8 months (ranging from 7 to 58 months). In the trial, severe immunosuppression with high doses of cyclophosphamide and anti-thymocyte globulin is used with the aim of "turning off" the immunologic system", and then autologous hematopoietic stem cells are reinfused to regenerate a new one. In summary it is a kind of "immunologic reset" that blocks the autoimmune attack against residual pancreatic insulin-producing cells. Until December 2009, 12 patients remained continuously insulin-free for periods ranging from 14 to 52 months and 8 patients became transiently insulin-free for periods ranging from 6 to 47 months. Of these last 8 patients, 2 became insulin-free again after the use of sitagliptin, a DPP-4 inhibitor approved only to treat type 2 diabetic patients and this is also the first study to document the use and complete insulin-independendce in humans with type 1 diabetes with this medication. In parallel with insulin suspension, indirect measures of endogenous insulin secretion revealed that it significantly increased in the whole group of patients, regardless the need of daily exogenous insulin use.[74]
Gene therapy

Technology for gene therapy is advancing rapidly such that there are multiple pathways possible to support endocrine function, with potential to practically cure diabetes.[75]
- Gene therapy can be used to manufacture insulin directly: an oral medication, consisting of viral vectors containing the insulin sequence, is digested and delivers its genes to the upper intestines. Those intestinal cells will then behave like any viral infected cell, and will reproduce the insulin protein. The virus can be controlled to infect only the cells which respond to the presence of glucose, such that insulin is produced only in the presence of high glucose levels. Due to the limited numbers of vectors delivered, very few intestinal cells would actually be impacted and would die off naturally in a few days. Therefore, by varying the amount of oral medication used, the amount of insulin created by gene therapy can be increased or decreased as needed. As the insulin-producing intestinal cells die off, they are boosted by additional oral medications.[76]
- Gene therapy might eventually be used to cure the cause of beta cell destruction, thereby curing the new diabetes patient before the beta cell destruction is complete and irreversible.[77]
- Gene therapy can be used to turn duodenum cells and duodenum adult stem cells into beta cells which produce insulin and amylin naturally. By delivering beta cell DNA to the intestine cells in the duodenum, a few intestine cells will turn into beta cells, and subsequently adult stem cells will develop into beta cells. This makes the supply of beta cells in the duodenum self replenishing, and the beta cells will produce insulin in proportional response to carbohydrates consumed.[78]
Type 2 diabetes
Type 2 diabetes is usually first treated by increasing physical activity, and eliminating saturated fat and reducing sugar and carbohydrate intake with a goal of losing weight. These can restore insulin sensitivity even when the weight loss is modest, for example around 5 kg (10 to 15 lb), most especially when it is in abdominal fat deposits. Diets that are very low in saturated fats have been claimed to reverse insulin resistance.[79][80]
Testosterone replacement therapy may improve glucose tolerance and insulin sensitivity in diabetic hypogonadal men. The mechanisms by which testosterone decreases insulin resistance is under study.[81] Moreover, testosterone may have a protective effect on pancreatic beta cells, which is possibly exerted by androgen-receptor-mediated mechanisms and influence of inflammatory cytokines.[82]
Recently it has been suggested that a type of gastric bypass surgery may normalize blood glucose levels in 80-100% of severely obese patients with diabetes. The precise causal mechanisms are being intensively researched; its results may not simply be attributable to weight loss, as the improvement in blood sugars seems to precede any change in body mass. This approach may become a treatment for some people with type 2 diabetes, but has not yet been studied in prospective clinical trials.[83] This surgery may have the additional benefit of reducing the death rate from all causes by up to 40% in severely obese people.[84] A small number of normal to moderately obese patients with type 2 diabetes have successfully undergone similar operations.[85][86]
MODY is another classification of diabetes and it can be treated by early lifesyle management and medical management. it has to be treated in the early stage, so as to provide a good health.
See also
- Ambulatory care sensitive conditions
- Diabetic coma
- Diabetic hypoglycemia
- Diabetic hypoglycemia journal
- Glycemic efficacy
References
- ↑ American Diabetes (January 2006). "Standards of medical care in diabetes--2006". Diabetes Care. 29. Suppl 1: S4–42. PMID 16373931. Retrieved 19 July 2008.
- ↑ Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK; Vijan; Snow; Cross; Weiss; Owens; Clinical Efficacy Assessment Subcommittee of the American College of Physicians (September 2007). "Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians". Annals of Internal Medicine 147 (6): 417–22. doi:10.7326/0003-4819-147-6-200709180-00012. PMID 17876024. Retrieved 19 July 2008.
- 1 2 3 American Diabetes Association (Jan 2014). "Standards of Medical Care in Diabetes 2014". Diabetes Care 37 (suppl 1): S14–S80. doi:10.2337/dc14-S014 (inactive 2015-01-12). PMID 24357209. Retrieved 1 Nov 2014.
- ↑ Brown AF, Mangione CM, Saliba D, Sarkisian CA; Mangione; Saliba; Sarkisian; California Healthcare Foundation/American Geriatrics Society Panel on Improving Care for Elders with Diabetes (May 2003). "Guidelines for improving the care of the older person with diabetes mellitus". J Am Geriatr Soc 51 (5 Suppl Guidelines): S265–80. doi:10.1046/j.1532-5415.51.5s.1.x. PMID 12694461. Retrieved 19 July 2008.
- 1 2 Walker, Donald (November 2007). "Similarity Determination and Case Retrieval in an Intelligent Decision Support System for Diabetes Management" (PDF). Retrieved 2 October 2009.
- ↑ "Blood Glucose Testing and Diabetes Management". TriMark Publications, LLC. May 2014.
- ↑ Arora, Karandeep Singh; Binjoo, Nagesh; Reddy, G. V. Ramachandra; Kaur, Prabhpreet; Modgil, Richa; Negi, Lalit Singh (2015-01-01). "Determination of normal range for fasting salivary glucose in Type 1 diabetics". Journal of International Society of Preventive & Community Dentistry 5 (5): 377–382. doi:10.4103/2231-0762.165923. ISSN 2231-0762. PMC 4606601. PMID 26539389.
- ↑ Zhang, HN; Lin, ZB (2004). "Hypoglycemic effect of Ganoderma lucidum polysaccharides". Acta pharmacologica Sinica 25 (2): 191–5. PMID 14769208.
- ↑ Yang, BK; Jung, YS; Song, CH (2007). "Hypoglycemic effects of Ganoderma applanatum and Collybia confluens exo-polymers in streptozotocin-induced diabetic rats". Phytotherapy Research 21 (11): 1066–9. doi:10.1002/ptr.2214. PMID 17600864.
- ↑ Konno, S; Tortorelis, DG; Fullerton, SA; Samadi, AA; Hettiarachchi, J; Tazaki, H (2001). "A possible hypoglycaemic effect of maitake mushroom on Type 2 diabetic patients". Diabetic medicine 18 (12): 1010. doi:10.1046/j.1464-5491.2001.00532-5.x. PMID 11903406.
- ↑ Hong, L; Xun, M; Wutong, W (2007). "Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice". The Journal of pharmacy and pharmacology 59 (4): 575–82. doi:10.1211/jpp.59.4.0013. PMID 17430642.
- ↑ Kubo, K; Aoki, H; Nanba, H (1994). "Anti-diabetic activity present in the fruit body of Grifola frondosa (Maitake). I". Biological & Pharmaceutical Bulletin 17 (8): 1106–10. doi:10.1248/bpb.17.1106. PMID 7820117.
- ↑ Lo, HC; Hsu, TH; Chen, CY (2008). "Submerged culture mycelium and broth of Grifola frondosa improve glycemic responses in diabetic rats". The American journal of Chinese medicine 36 (2): 265–85. doi:10.1142/S0192415X0800576X (inactive 2015-01-12). PMID 18457360.
- ↑ Manohar, V; Talpur, NA; Echard, BW; Lieberman, S; Preuss, HG (2002). "Effects of a water-soluble extract of maitake mushroom on circulating glucose/insulin concentrations in KK mice". Diabetes, obesity & metabolism 4 (1): 43–8. doi:10.1046/j.1463-1326.2002.00180.x. PMID 11874441.
- ↑ Horio, H; Ohtsuru, M (2001). "Maitake (Grifola frondosa) improve glucose tolerance of experimental diabetic rats". Journal of nutritional science and vitaminology 47 (1): 57–63. doi:10.3177/jnsv.47.57 (inactive 2015-01-12). PMID 11349892.
- ↑ Liu, Y; Fukuwatari, Y; Okumura, K; Takeda, K; Ishibashi, KI; Furukawa, M; Ohno, N; Mori, K; et al. (2008). "Immunomodulating Activity of Agaricus brasiliensis KA21 in Mice and in Human Volunteers". Evidence-based complementary and alternative medicine 5 (2): 205–219. doi:10.1093/ecam/nem016. PMC 2396466. PMID 18604247.
- ↑ Kim, YW; Kim, KH; Choi, HJ; Lee, DS (2005). "Anti-diabetic activity of beta-glucans and their enzymatically hydrolyzed oligosaccharides from Agaricus blazei". Biotechnology letters 27 (7): 483–7. doi:10.1007/s10529-005-2225-8. PMID 15928854.
- ↑ Hsu, CH; Liao, YL; Lin, SC; Hwang, KC; Chou, P (2007). "The mushroom Agaricus Blazei Murill in combination with metformin and gliclazide improves insulin resistance in type 2 diabetes: a randomized, double-blinded, and placebo-controlled clinical trial". Journal of alternative and complementary medicine 13 (1): 97–102. doi:10.1089/acm.2006.6054. PMID 17309383.
- ↑ Fortes, RC; Novaes, MR; Recôva, VL; Melo, AL (2009). "Immunological, hematological, and glycemia effects of dietary supplementation with Agaricus sylvaticus on patients' colorectal cancer". Experimental biology and medicine 234 (1): 53–62. doi:10.3181/0806-RM-193. PMID 18997106.
- ↑ Briscoe, V. J. (2006). "Hypoglycemia in Type 1 and Type 2 Diabetes: Physiology, Pathophysiology, and Management". Clinical Diabetes 24 (3): 115–121. doi:10.2337/diaclin.24.3.115.
- ↑ Fishbein, H.; Palumbo, P. (1995). "Acute Metabolic Complications in Diabetes". Diabetes in America. Bethesda: National Diabetes Data Group. p. 283.
- ↑ Asuncion, MM; Shaheen, M; Ganesan, K; Velasques, J; Teklehaimanot, S; Pan, D; Norris, K (2007). "Increase in hypoglycemic admissions: California hospital discharge data". Ethnicity & disease 17 (3): 536–40. PMID 17985510.
- ↑ Perlmuter, LC; Flanagan, BP; Shah, PH; Singh, SP (2008). "Glycemic Control and Hypoglycemia: Is the loser the winner?". Diabetes Care 31 (10): 2072–6. doi:10.2337/dc08-1441. PMC 2551657. PMID 18820231.
- ↑ Tupola, S; Rajantie, J; Mäenpää, J (1998). "Severe hypoglycaemia in children and adolescents during multiple-dose insulin therapy". Diabetic medicine 15 (8): 695–9. doi:10.1002/(SICI)1096-9136(199808)15:8<695::AID-DIA651>3.0.CO;2-C. PMID 9702475.
- ↑ Fujioka, M; Okuchi, K; Hiramatsu, KI; Sakaki, T; Sakaguchi, S; Ishii, Y (1997). "Specific changes in human brain after hypoglycemic injury". Stroke; a journal of cerebral circulation 28 (3): 584–7. doi:10.1161/01.STR.28.3.584. PMID 9056615.
- 1 2 Sugimoto, K; Baba, M; Suda, T; Yasujima, M; Yagihashi, S (2003). "Peripheral neuropathy and microangiopathy in rats with insulinoma: association with chronic hyperinsulinemia". Diabetes/metabolism research and reviews 19 (5): 392–400. doi:10.1002/dmrr.395. PMID 12951647.
- ↑ Tarnow, L; Groop, PH; Hadjadj, S; Kazeem, G; Cambien, F; Marre, M; Forsblom, C; Parving, HH; et al. (2008). "European rational approach for the genetics of diabetic complications--EURAGEDIC: patient populations and strategy". Nephrology, dialysis, transplantation 23 (1): 161–8. doi:10.1093/ndt/gfm501. PMID 17704113.
- ↑ Adams, DD (2008). "Autoimmune destruction of pericytes as the cause of diabetic retinopathy". Clinical ophthalmology 2 (2): 295–8. doi:10.2147/OPTH.S2629. PMC 2693966. PMID 19668719.
- ↑ Huang, ES; Brown, SE; Ewigman, BG; Foley, EC; Meltzer, DO (2007). "Patient Perceptions of Quality of Life With Diabetes-Related Complications and Treatments". Diabetes Care 30 (10): 2478–83. doi:10.2337/dc07-0499. PMC 2288662. PMID 17623824.
- ↑ http://www.ncbi.nlm.nih.gov/pubmed/?term=22212499
- ↑ Evans, Josie M M; Newton, Ray W; Ruta, Danny A; MacDonald, Thomas M; Stevenson, Richard J; Morris, Andrew D (1999). "Frequency of blood glucose monitoring in relation to glycaemic control: observational study with diabetes database". BMJ 319 (7202): 83–6. doi:10.1136/bmj.319.7202.83. PMC 28155. PMID 10398627.
- ↑ Gallichan, M (1997). "Self monitoring of glucose by people with diabetes: evidence based practice". BMJ 314 (7085): 964–7. doi:10.1136/bmj.314.7085.964. PMC 2126360. PMID 9099125.
- ↑ Chantelau, E; Nowicki, S (1997). "Self monitoring of glucose by people with diabetes. Patients with non-insulin dependent diabetes should monitor urine urine rather than blood glucose". BMJ 315 (7101): 185. doi:10.1136/bmj.315.7101.184. PMC 2127136. PMID 9251556.
- ↑ Farmer, A; Wade, A; French, DP; Goyder, E; Kinmonth, AL; Neil, A (2005). "The DiGEM trial protocol – a randomised controlled trial to determine the effect on glycaemic control of different strategies of blood glucose self-monitoring in people with type 2 diabetes ISRCTN47464659". BMC family practice 6 (1): 25. doi:10.1186/1471-2296-6-25. PMC 1185530. PMID 15960852.
- ↑ Kibriya, MG; Ali, L; Banik, NG; Khan, AK (1999). "Home monitoring of blood glucose (HMBG) in Type-2 diabetes mellitus in a developing country". Diabetes research and clinical practice 46 (3): 253–7. doi:10.1016/S0168-8227(99)00093-5. PMID 10624792.
- ↑ Jaworska, J; Dziemidok, P; Kulik, TB; Rudnicka-Drozak, E (2004). "Frequency of self-monitoring and its effect on metabolic control in patients with type 2 diabetes". Annales Universitatis Mariae Curie-Sklodowska. Sectio D: Medicina 59 (1): 310–6. PMID 16146003.
- ↑ Roach, P (2004). "Better systems, not guidelines, for glucose monitoring". BMJ 329 (7479): E332. doi:10.1136/bmj.329.7479.E332. PMID 15591539.
- ↑ "Dietary carbohydrate restriction". nutritionjrnl. nutritionjrnl.com. Retrieved 14 November 2015.
- ↑ http://www.dmsjournal.com/content/4/1/23
- ↑ http://www.mountsinai.org/about-us/newsroom/press-releases/diabetic-kidney-failure-may-be-reversed-with-low-carbohydrate-diet
- ↑ http://www.dmsjournal.com/content/pdf/1758-5996-4-23.pdf
- ↑ http://www.abc.net.au/radionational/programs/healthreport/low-carbohydrate-diet-to-manage-diabetes/4880362
- ↑ http://www.abc.net.au/catalyst/stories/4126228.htm
- ↑ http://www.diabetes.co.uk/diet-for-type1-diabetes.html
- ↑ http://www.fshn.chhs.colostate.edu/outreach/lfs/files/2013-presentations/8-Volek-Moving%20Toward%20a%20Personalized%20Approach%20to%20Nutrition.pdf
- ↑ http://www.uab.edu/news/innovation/item/4997-low-carb-diet-recommended-for-diabetics
- ↑ Tuomilehto, J; Lindström, J; Eriksson, JG; Valle, TT; Hämäläinen, H; Ilanne-Parikka, P; Keinänen-Kiukaanniemi, S; Laakso, M; et al. (2001). "Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance". The New England Journal of Medicine 344 (18): 1343–50. doi:10.1056/NEJM200105033441801. PMID 11333990.
- ↑ Oramed Pharmaceuticals. "Making insulin delivery in capsule form a reality".
- ↑ Mealey, Brian L. (2006). "Diabetes Mellitus Management". Diabetes Mellitus and Oral Health. Armenian Medical Network. Retrieved 2 October 2009.
- ↑ Alexander, G Caleb; Sehgal NL; Moloney RM; Stafford RS (27 October 2008). "National trends in treatment of type 2 diabetes mellitus, 1994-2007". Archives of Internal Medicine 168 (19): 2088–2094. doi:10.1001/archinte.168.19.2088. PMC 2868588. PMID 18955637.
- ↑ Mudaliar, S (2009). "Serum glucose control in diabetic patients with cardiovascular disease: should we be less aggressive?". Current atherosclerosis reports 11 (5): 384–90. doi:10.1007/s11883-009-0058-y. PMID 19664383.
- ↑ Songer, TJ. Low blood sugar and motor vehicle crashes in persons with type 1 diabetes, Annu Proc Assoc Adv Automotive Med, 46:424-427 (2002)
- ↑ Cox DJ, Penberthy JK, Zrebiec J, Weinger K, Aikens JE, Frier BM, Stetson B, DeGroot M, Trief P; et al. (2003). "Diabetes and Driving Mishaps: Frequency and correlations from a multinational survey". Diabetes Care 26 (8): 2329–2334. doi:10.2337/diacare.26.8.2329. PMID 12882857.
- 1 2 Cox DJ, Gonder-Frederick LA, Clarke WL (1993). "Driving decrements in type 1 diabetes during moderate hypoglycemia". Diabetes 42 (2): 239–243. doi:10.2337/diabetes.42.2.239. PMID 8425660.
- ↑ Clarke WL, Cox DJ, Gonder-Frederick LA, Kovatchev B (1999). "Hypoglycemia and the Decision to Drive a Motor Vehicle by Persons With Diabetes". JAMA 282 (8): 750–754. doi:10.1001/jama.282.8.750. PMID 10463710.
- ↑ Cox D, Gonder-Frederick LA, Kovatchev BP, Julian DM, Clarke WL (2000). "Progressive hypoglycemia's impact on driving simulation performance". Diabetes Care 23 (2): 163–170. doi:10.2337/diacare.23.2.163. PMID 10868825.
- 1 2 Cox DJ, Kovatchev BP, Anderson SM, Clarke WL, Gonder-Frederick LA (November 2010). "Type 1 diabetic drivers with and without a history of recurrent hypoglycemia-related driving mishaps: physiological and performance differences during euglycemia and the induction of hypoglycemia". Diabetes Care 33 (11): 2430–5. doi:10.2337/dc09-2130. PMC 2963507. PMID 20699432.
- ↑ Cox DJ, Gonder-Frederick LA, Kovatchev BP, Clarke WL (2002). "The metabolic demands of driving for drivers with type 1 diabetes mellitus". Diabetes/Metabolism Research and Review 18 (5): 381–385. doi:10.1002/dmrr.306.
- ↑ Campbell LK, Gonder-Frederick LA, Broshek DK, Kovatchev BP, Anderson S, Clarke WL, Cox DJ (2010). "Neurocognitive differences between drivers with type 1 diabetes with and without a recent history of recurrent driving mishaps". International Journal of Diabetes 2 (2): 73–77. doi:10.1016/j.ijdm.2010.05.014.
- ↑ Cox DJ, Gonder-Frederick LA, Julian D, Clarke W (1994). "Long-term follow-up evaluation of blood glucose awareness training". Diabetes Care 17 (1): 1–5. doi:10.2337/diacare.17.1.1. PMID 8112183.
- ↑ Cox DJ, Gonder-Frederick LA, Polonsky W, Schlundt D, Julian D, Kovatchev B, Clarke WL (2001). "Blood Glucose Awareness Training (BGAT-II): Long term benefits". Diabetes Care 24 (4): 637–642. doi:10.2337/diacare.24.4.637. PMID 11315822.
- ↑ Broers S., Cessie S., van Vliet KP, Spinhoven P., der Ven NC, Radder JK (2002). "Blood glucose awareness training in Dutch type 1 diabetes patients". Diabet. Med. 19 (2): 157–161. doi:10.1046/j.1464-5491.2002.00682.x. PMID 11874433.
- ↑ Cox DJ, Ritterband L, Magee J, Clarke W, Gonder-Frederick L (2008). "Blood Glucose Awareness Training Delivered Over The Internet". Diabetes Care 31 (8): 1527–1528. doi:10.2337/dc07-1956. PMC 2494647. PMID 18477813.
- ↑ http://www.DiabetesDriving.com Diabetes Driving.
- ↑ "Cindy Marling".
- ↑ "Oral diabetes care". Retrieved 2010-05-05.
- 1 2 "Gum Disease and Diabetes". Retrieved 2010-05-05.
- ↑ Koh GCKW, van der Poll T, Peacock SJ (2011). "The impact of diabetes on the pathogenesis of sepsis". Eur J Clin Microbiol & Infect Dis 31 (4): 379–88. doi:10.1007/s10096-011-1337-4. PMC 3303037. PMID 21805196.
- ↑ "Diabetes and Dental Care: Guide to a Healthy Mouth". Retrieved 2010-05-05.
- ↑ "Diabetes and Oral Health". Retrieved 2010-05-05.
- ↑ Chan M (2010). "Reducing cost-related medication nonadherence in patients with diabetes". Drug Benefit Trends 22: 67–71.
- ↑ Cerco Medical: Science: Methods
- ↑ Voltarelli JC, Couri CE, Stracieri AB, et al. (2007). "Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA 297 (14): 1568–76. doi:10.1001/jama.297.14.1568. PMID 17426276.
- ↑ Couri CE, Oliveira MC, Stracieri AB, et al. (April 2009). "C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus". JAMA 301 (15): 1573–9. doi:10.1001/jama.2009.470. PMID 19366777.
- ↑ Gene Therapy Approaches to Diabetes
- ↑ Mary Ann Liebert, Inc. - Cookie absent
- ↑ hopkinsbayview.org
- ↑ Engene Inc
- ↑ Barnard, Neal (2007). "13". Dr. Neal Barnard's Program for Reversing Diabetes: The Scientifically Proven System for Reversing Diabetes Without Drugs. New York, NY: Rodale/Holtzbrinck Publishers. ISBN 978-1-59486-528-2.
- ↑ Barnard ND, Katcher HI, Jenkins DJ, Cohen J, Turner-McGrievy G; Katcher; Jenkins; Cohen; Turner-Mcgrievy (May 2009). "Vegetarian and vegan diets in type 2 diabetes management". Nutrition Reviews 67 (5): 255–63. doi:10.1111/j.1753-4887.2009.00198.x (inactive 2015-01-12). PMID 19386029.
- ↑ Traish AM, Saad F, Guay A; Saad; Guay (2009). "The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance". Journal of Andrology 30 (1): 23–32. doi:10.2164/jandrol.108.005751. PMID 18772488.
- ↑ Zitzmann M (October 2009). "Testosterone deficiency, insulin resistance and the metabolic syndrome". Nature Reviews Endocrinology 5 (12): 673–81. doi:10.1038/nrendo.2009.212. PMID 19859074.
- ↑ Rubino F, Gagner M; Gagner (November 2002). "Potential of Surgery for Curing Type 2 Diabetes Mellitus". Annals of Surgery 236 (5): 554–9. doi:10.1097/01.SLA.0000032951.37471.80 (inactive 2015-01-12). PMC 1422611. PMID 12409659.
- ↑ Adams TD, Gress RE, Smith SC; et al. (August 2007). "Long-term mortality after gastric bypass surgery". The New England Journal of Medicine 357 (8): 753–61. doi:10.1056/NEJMoa066603. PMID 17715409.
- ↑ Cohen RV, Schiavon CA, Pinheiro JS, Correa JL, Rubino F; Schiavon; Pinheiro; Correa; Rubino (2007). "Duodenal-jejunal bypass for the treatment of type 2 diabetes in patients with body mass index of 22-34 kg/m2: a report of 2 cases". Surgery for Obesity and Related Diseases 3 (2): 195–7. doi:10.1016/j.soard.2007.01.009. PMID 17386401.
- ↑ Vasonconcelos, Alberto (1 September 2007). "Could type 2 diabetes be reversed using surgery?". New Scientist (2619): 11–3. Retrieved 26 September 2007.
External links
- Friend of Diabetic : Online Diabetes Management Software
- Alliance to Reduce Disparities in Diabetes
- Center for Managing Chronic Disease, University of Michigan
- American College of Physicians Diabetes Portal - Resources for patients and clinicians
- American Diabetes Association
- Prevent Diabetes Problems: Keep Your Diabetes Under Control - Self-care tips at the United States "National Diabetes Information Clearinghouse"
- http://faculty.ksu.edu.sa/Alhowikan/Documents/Exercise and diabetes Mellitus alhowikan.ppt A helpful presentation that highlights the importance of exercise in the prevention of diabetes.
- Latent autoimmune diabetes of adults Autoimmune component of late onset or type two diabetes.
| ||||||||||||||||||||||