Trazodone
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
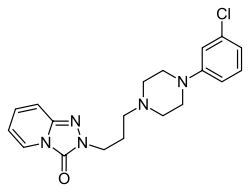
2-{3-[4-(3-chlorophenyl)piperazin-1-yl]propyl}[1,2,4]triazolo[4,3-a]pyridin-3(2H)-one | |
| Clinical data | |
| Trade names | Many brand names worldwide[1] |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a681038 |
| Pregnancy category |
|
| Legal status | |
| Routes of administration | Oral |
| Pharmacokinetic data | |
| Bioavailability | 65% (Oral)[2] |
| Protein binding | 89–95%[3] |
| Metabolism | Hepatic (CYP3A4)[2] |
| Onset of action | 1 hour (oral)[4] |
| Biological half-life |
7 hours (immediate-release), 10 hours (extended-release)[2] |
| Excretion |
21% faeces, 70–75% urine[2] |
| Identifiers | |
| CAS Number |
19794-93-5 |
| ATC code | N06AX05 |
| PubChem | CID 5533 |
| IUPHAR/BPS | 213 |
| DrugBank |
DB00656 |
| ChemSpider |
5332 |
| UNII |
YBK48BXK30 |
| KEGG |
D08626 |
| ChEBI |
CHEBI:9654 |
| ChEMBL |
CHEMBL621 |
| Chemical data | |
| Formula | C19H22ClN5O |
| Molar mass | 371.8641 g/mol |
| |
| |
| (verify) | |
Trazodone (sold under many brand names worldwide[1]) is an antidepressant of the serotonin antagonist and reuptake inhibitor (SARI) class. It is a phenylpiperazine compound. Trazodone also has antianxiety (anxiolytic) and sleep-inducing (hypnotic) effects.[5] Its side-effect profile and potential toxicity are considerably different from those of the original antidepressants (i.e., the monoamine oxidase inhibitors (MAOIs) and tricyclic antidepressants (TCAs)).[6]
Medical uses
- Anxiety disorder[7]
- Unipolar depression, with or without anxiety[8]
- Insomnia[9][10][11] (in some countries, this is an off-label use)
Off-label and investigational uses
- Fibromyalgia[12]
- Complex regional pain syndrome[13]
- Control of nightmares[14]
- Panic disorder[15]
- Diabetic neuropathy[16]
- Bulimia nervosa[17]
- Obsessive-compulsive disorder (OCD)[18][19]
- Alcohol withdrawal[20][21][22]
- Schizophrenia as an adjunct to improve negative symptoms[23]
- Erectile dysfunction[24]
The primary use of trazodone is the treatment of major depression. Data from open and double-blind trials suggest the antidepressant efficacy of trazodone is comparable to that of amitriptyline, doxepin, and mianserin. Also, trazodone showed anxiolytic properties, low cardiotoxicity, and relatively mild side effects.[6] Because trazodone has minimal anticholinergic activity, it was especially welcomed as a treatment for geriatric patients with depression when it first became available. Three double-blind studies reported trazodone has antidepressant efficacy similar to that of other antidepressants in geriatric patients. However, a side effect of trazodone, orthostatic hypotension, which may cause dizziness and increase the risk of falling, can have devastating consequences for elderly patients; thus, this side effect, along with sedation, often makes trazodone less acceptable for this population, compared with newer compounds that share its lack of anticholinergic activity but not the rest of its side-effect profile. Still, trazodone is often helpful for geriatric patients with depression who have severe agitation and insomnia.[6] Trazodone has also been reported to have antianxiety properties. In a randomized, double-blind, placebo-controlled trial, the anxiolytic efficacy of trazodone was comparable to that of diazepam in weeks 3–8 of treatment for generalized anxiety disorder, although patients treated with diazepam had greater improvement during the first 2 weeks of treatment.[16] Early case reports had indicated that trazodone is associated with improvement in obsessive-compulsive disorder, but a double-blind, placebo-controlled study found that trazodone lacked antiobsessional effects.[19] Many clinicians use low-dose trazodone as an alternative to benzodiazepines for the treatment of insomnia. Two recent reviews found that trazodone is the second most prescribed agent for insomnia, but as most studies have been limited to patients with depression, few studies actually support trazodone's use in primary insomnia.[6]
Adverse effects
Because of its lack of anticholinergic side effects, trazodone is especially useful in situations in which antimuscarinic effects are particularly problematic (e.g., in patients with benign prostatic hyperplasia, closed-angle glaucoma, or severe constipation). Trazodone's propensity to cause sedation is a dual-edged sword. For many patients, the relief from agitation, anxiety, and insomnia can be rapid; for other patients, including those individuals with considerable psychomotor retardation and feelings of low energy, therapeutic doses of trazodone may not be tolerable because of sedation. Trazodone elicits orthostatic hypotension in some patients, probably as a consequence of 1-adrenergic receptor blockade. Mania has been observed in association with trazodone treatment, including in patients with bipolar disorder, as well as in patients with previous diagnoses of major depression. Compared to the reversible MAOI antidepressant drug moclobemide, significantly more impairment of vigilance occurs with trazodone.[25]
Cardiac arrhythmia
Case reports have noted cardiac arrhythmias emerging in relation to trazodone treatment, both in patients with pre-existing mitral valve prolapse and in patients with negative personal and family histories of cardiac disease.[26]
QT prolongation has been reported with trazodone therapy. Arrhythmia identified include isolated PVCs, ventricular couplets, and in two patients short episodes (three to four beats) of ventricular tachycardia. Several post-marketing reports have been made of arrhythmia in trazodone-treated patients who have pre-existing cardiac disease and in some patients who did not have pre-existing cardiac disease. Until the results of prospective studies are available, patients with pre-existing cardiac disease should be closely monitored, particularly for cardiac arrhythmias. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction. Concomitant administration of drugs that prolong the QT interval or that are inhibitors of CYP3A4 may increase the risk of cardiac arrhythmia.
Priapism
A relatively rare, but dramatic, side effect associated with trazodone is priapism, likely due to its antagonism at α-adrenergic receptors.[27] Priapism is a potentially harmful medical condition in which the erect penis does not return to its flaccid state (despite the absence of both physical and psychological stimulation) within four hours.[28] More than 200 cases have been reported, and the manufacturer estimated that the incidence of any abnormal erectile function is about one in 6,000 male patients treated with trazodone. The risk for this side effect appears to be greatest during the first month of treatment at low dosages (i.e. <150 mg/day). Early recognition of any abnormal erectile function is important, including prolonged or inappropriate erections, and should prompt discontinuation of trazodone treatment. Clinical reports have also described trazodone-associated psychosexual side effects in women, including increased libido, priapism of the clitoris, and spontaneous orgasms.[26][29]
Other
Rare cases of idiosyncratic hepatotoxicity have been observed, possibly due to the formation of reactive metabolites.[30]
Elevated prolactin concentrations have been observed in patients taking trazodone.[31]
Precautions
- If the patient has a known hypersensitivity to trazodone
- If the patient is under 18 years of age and trazodone is combined with other antidepressant medications, it may increase the possibility of suicidal thoughts or actions.[32]
Trazodone is metabolized by CYP3A4, a liver enzyme.[33] Inhibition of this enzyme by various other substances may delay its degradation, leading to high blood levels of trazodone. CYP3A4 may be inhibited by many other medications, herbs, and foods, and as such, trazodone may interact with these substances. One drug-food interaction is grapefruit juice. Drinking grapefruit juice is discouraged in patients taking trazodone. One glass of grapefruit juice occasionally is not likely to have this effect on most people, but drinking large amounts, or drinking it regularly is proven to affect trazodone's clearance.
The possibility of suicide in depressed patients remains during treatment and until significant remission occurs. The number of tablets prescribed at any one time should take into account this possibility, and patients with suicidal ideation should never have access to large quantities of trazodone.
Trazodone has been reported to cause seizures in a small number of patients who took it concurrently with medications to control seizures.
While trazodone is not a true member of the SSRI class of antidepressants, it does still share many properties of the SSRIs, especially the possibility of discontinuation syndrome if the medication is stopped too quickly.[34] Care must, therefore, be taken when coming off the medication, usually by a gradual process of tapering down the dose over a period of time.
Pregnancy and lactation
Sufficient data in humans are lacking. Use should be justified by the severity of the condition to be treated.[35][36]
Occupational hazards
Since trazodone may impair the mental and/or physical abilities required for performance of potentially hazardous tasks, such as operating an automobile or machinery, the patient should be cautioned not to engage in such activities while impaired.
Overdose
There are reported cases of high doses of trazodone precipitating serotonin syndrome.[37] There are also reports of patients taking multiple SSRIs with trazodone and precipitating serotonin syndrome.[38]
Signs and symptoms
Trazodone appears to be relatively safer than TCAs, MAOIs, and a few of the other second-generation antidepressants in overdose situations, especially when it is the only agent taken. Fatalities are rare, and uneventful recoveries have been reported after ingestion of doses as high as 6,000–9,200 mg. In one report, 9 of 294 cases of overdose were fatal, and all 9 patients had also taken other central nervous system (CNS) depressants. When trazodone overdoses occur, clinicians should carefully monitor for hypotension, a potentially serious toxic effect. In a report of a fatal trazodone overdose, torsades de pointes and complete atrioventricular block developed, along with subsequent multiple organ failure, with a trazodone plasma concentration of 25.4 mg/L on admission.[6][39][40][41]
Treatment
There is no specific antidote for trazodone. Management of overdosage should, therefore, be symptomatic and supportive. Any person suspected of having taken an overdosage should be evaluated at a hospital as soon as possible. Activated charcoal, and forced diuresis may be useful in facilitating elimination of the drug, gastric lavage has been show to not be useful unless done during the first hour after intake.
Pharmacology
Binding profile
Trazodone behaves as an antagonist at all of its receptor sites except 5-HT1A, where it acts as a partial agonist similarly to buspirone and tandospirone, but with greater intrinsic activity in comparison.[42][43][44][45][46][47]
It is an inhibitor of Serotonin transporters (SERT), as well.[48]
Binding affinity (Ki [nM]) towards cloned human receptors where data are available
| Protein | Ki (nM) for trazodone[49] | Ki (nM) for mCPP |
|---|---|---|
| SERT | 367.3 | 265.1 |
| NET | >10000 | 3150 |
| DAT | >7000 | ND |
| 5-HT1A | 118 | 43.9 |
| 5-HT1B | >10000 | 251.9 |
| 5-HT1D | 106 | 285.5 |
| 5-HT1E | >10000 | ND |
| 5-HT2A | 35.8 | 54.5 |
| 5-HT2B | 78.4 | 30.3 |
| 5-HT2C | 223.9 | 13.04 |
| 5-HT3 | >10000 | 427 |
| 5-HT5A | >10000 | 1354 |
| 5-HT6 | >10000 | 1748 |
| 5-HT7 | 1782 | 162.5 |
| α1A | 153 | 1386 |
| α1B | ND | 914.6 |
| α2A | 728 | 145.1 |
| α2B | ND | 105.8 |
| α2C | 155 | 123.5 |
| β1 | >10000 | 2359 |
| β2 | >10000 | 3474 |
| D1 | 3730 | 7000 (HB) |
| D2 | 4142 | >10000 (HB) |
| D3 | ND | >10000 (RC) |
| D4 | 703 | ND |
| D5 | >10000 | >10000 |
| Benzodiazepine receptor | >10000 (RK,P) | >10000 (HB,C) |
| Calcium channel | >10000 | >10000 |
| Cannabinoid receptors | >10000 | ND |
| I1 | ND | 758.9 (RPC12) |
| σ1 | >10000 | ND |
| σ2 | 536 (RPC12) | 8350 (RPC12) |
| H1 | 220 | 326.3 |
| H2 | 3290 | ND |
| H3 | >10000 (GPC) | ND |
| H4 | >10000 | ND |
| mAChRs | >10000 | >10000 (HB) |
| nAChRs | >10000 | ND |
| μ-opioid | >10000 | ND |
| δ-opioid | >10000 | ND |
| κ-opioid | >10000 (RC) | ND |
| E3 | >10000 | ND |
| E4 | >10000 | ND |
Legend:
ND - No data
GPC - Cloned guinea pig receptor.
P - Peripheral receptor
C - Central receptor
RC - Cloned rat receptor
RK - Rat kidney receptor
HB - Human brain receptor
RPC12 - Rat PC12 receptor
Bold text indicates that binding affinities for cloned human receptors is unavailable.
Correspondence to clinical effects
Trazodone acts predominantly as a 5-HT2A receptor antagonist to mediate its therapeutic benefits against anxiety and depression.[50] Its inhibitory effects on serotonin reuptake and 5-HT2C receptors are relatively weak (~15-fold lower than for 5-HT2A) and contribute only lightly to its overall effects.[50] Hence, trazodone does not have similar properties to selective serotonin reuptake inhibitors (SSRIs)[50] and is not particularly associated with increased appetite and weight gain, unlike other 5-HT2C antagonists like mirtazapine.[51][52] Moderate 5-HT1A partial agonism (6-fold lower than 5-HT2A) is likely to contribute to trazodone's antidepressant and anxiolytic actions to some extent as well.[46][47][53]
The combined actions of 5HT2A/5HT2C antagonism with SERT inhibition only occur at moderate to high doses of trazodone. Doses of trazodone lower than those effective for antidepressant action are frequently used for the effective treatment of insomnia. Low doses exploit trazodone’s potent actions as a 5HT2A antagonist, and its properties as an antagonist of H1-histaminic and α1-adrenergic receptors, but do not adequately exploit its SERT or 5HT2C inhibition properties, which are weaker. Since insomnia is one of the most frequent residual symptoms of depression after treatment with an SSRI, a hypnotic is often necessary for patients with a major depressive episode. Not only can a hypnotic potentially relieve the insomnia itself, but treating insomnia in patients with major depression may also increase remission rates due to improvement of other symptoms such as loss of energy and depressed mood. Thus, the ability of low doses of trazodone to improve sleep in depressed patients may be an important mechanism whereby trazodone can augment the efficacy of other antidepressants.[54]
Trazodone's potent α1-adrenergic blockade (about threefold lower relative to 5-HT2A) may cause some side effects like orthostatic hypotension and sedation.[55] Conversely, along with 5-HT2A antagonism, it may underlie its efficacy as a hypnotic. This seems possible as trazodone's antihistamine activity is relatively weak and probably clinically insignificant; hence, it cannot explain trazodone's sleep-inducing/enhancing effects. Trazodone lacks any affinity for the mACh receptors, so does not produce anticholinergic side effects.
mCPP, a nonselective serotonin receptor agonist and serotonin releasing agent, is an active metabolite of trazodone and has been suggested to possibly play a role in its therapeutic benefits.[56][57][58] However, scientific research has not supported this hypothesis, and mCPP may actually antagonize trazodone's efficacy as well as produce additional side effects.[59][60][61][62][63]
Pharmacokinetics
Trazodone is well absorbed after oral administration, with mean peak blood levels obtained at about one hour after ingestion. Absorption is somewhat delayed and enhanced by food. The mean blood elimination half-life is biphasic: the first phase's half-life is 3–6 hr, and the following phase's half-life is 5–9 hr. The drug is extensively metabolized, with three or four major metabolites having been identified in the human body, particularly mCPP,[33] which may contribute to the side effect profile of trazodone. mCPP has been shown to activate numerous serotonin receptors, including 5-HT2C. Around 70–75% of 14C-labelled trazodone was found to be excreted in the urine within 72 hours.[64] Trazodone is highly protein-bound.
As a consequence of the production of mCPP as a metabolite, patients administered trazodone may test positive on EMIT II urine tests for the presence of MDMA ("ecstasy").[65]
Forms of trazodone
Trazodone tablets are available in 50, 100, 150 and 300 mg strengths. However, the less-frequently prescribed 300 mg strength has been widely discontinued by many pharmaceutical manufacturers as of mid-2015. The active ingredient is trazodone hydrochloride, USP.
Inactive ingredients vary by manufacturer. See manufacturer package insert for more information.[66]
History
Trazodone was originally discovered and developed in Italy in the 1960s by Angelini Research Laboratories as a second-generation antidepressant. It was developed according to the mental pain hypothesis, which was postulated from studying patients and which proposes that major depression is associated with a decreased pain threshold.[67] In sharp contrast to most other antidepressants available at the time of its development, trazodone showed minimal effects on muscarinic cholinergic receptors. Trazodone was patented and marketed in many countries all over the world. It was approved by the Food and Drug Administration (FDA) at the end of 1981.
See also
References
- 1 2 Drugs.com international listings for Trazodone Page accessed Feb 10, 2016
- 1 2 3 4 Truven Health Analytics, Inc. DrugPoint® System (Internet) [cited 2013 Oct 1]. Greenwood Village, CO: Thomsen Healthcare; 2013.
- ↑ DrugBank http://www.drugbank.ca/drugs/DB00656. Retrieved 7 June 2015. Missing or empty
|title=(help) - ↑ MicroMedex DrugPoints. Missing or empty
|title=(help); - ↑ Haria M, Fitton A, McTavish D (April 1994). "Trazodone. A review of its pharmacology, therapeutic use in depression and therapeutic potential in other disorders". Drugs Aging 4 (4): 331–55. doi:10.2165/00002512-199404040-00006. PMID 8019056.
- 1 2 3 4 5 Schatzberg, AF; Nemeroff, CB, eds. (2009). Textbook of Psychopharmacology (4th ed.). Washington, D.C.: American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- ↑ British National Formulary (BNF) 65. London, UK: Pharmaceutical Press. 2013. p. 247. ISBN 9780857110848.
- ↑ "Desyrel - FDA Prescribing Information". Drugs.com. Retrieved 4 June 2015.
- ↑ Nierenberg AA, Adler LA, Peselow E, Zornberg G, Rosenthal M (July 1994). "Trazodone for antidepressant-associated insomnia". Am J Psychiatry 151 (7): 1069–72. doi:10.1176/ajp.151.7.1069. PMID 8010365.
- ↑ Kaynak H, Kaynak D, Gözükirmizi E, Guilleminault C (January 2004). "The effects of trazodone on sleep in patients treated with stimulant antidepressants". Sleep Med. 5 (1): 15–20. doi:10.1016/j.sleep.2003.06.006. PMID 14725822.
- ↑ Scharf MB, Sachais BA (September 1990). "Sleep laboratory evaluation of the effects and efficacy of trazodone in depressed insomniac patients". J Clin Psychiatry 51 (Suppl): 13–7. PMID 2211559.
- ↑ Morillas-Arques, P.; Rodriguez-Lopez, C.; Molina-Barea, R.; Rico-Villademoros, F.; Calandre, E. P. (2010). "Trazodone for the treatment of fibromyalgia: An open-label, 12-week study". BMC Musculoskeletal Disorders 11: 204. doi:10.1186/1471-2474-11-204. PMC 2945951. PMID 20831796.
- ↑ "Understanding the Pharmacologic Therapy for Complex Regional Pain Syndrome: Pharmacologic Therapy". Medscape.com. Retrieved 2014-03-14.
- ↑ Warner,, M. D.,; Dorn,, M. R.,; Peabody, C. A., (2001). "Survey on the Usefulness of Trazodone in Patients with PTSD with Insomnia or Nightmares journal=Pharmacopsychiatry" 34 (4): 128–131. doi:10.1055/s-2001-15871. PMID 11518472
- ↑ Mavissakalian M, Perel J, Bowler K, Dealy R; Perel; Bowler; Dealy (June 1987). "Trazodone in the treatment of panic disorder and agoraphobia with panic attacks". Am J Psychiatry 144 (6): 785–7. doi:10.1176/ajp.144.6.785. PMID 3296792.
- 1 2 Rickels K, Downing R, Schweizer E, Hassman H; Downing; Schweizer; Hassman (November 1993). "Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam". Arch. Gen. Psychiatry 50 (11): 884–95. doi:10.1001/archpsyc.1993.01820230054005. PMID 8215814.
- ↑ Pope HG, Keck PE, McElroy SL, Hudson JI; Keck Jr; McElroy; Hudson (August 1989). "A placebo-controlled study of trazodone in bulimia nervosa". J Clin Psychopharmacol 9 (4): 254–9. doi:10.1097/00004714-198908000-00004. PMID 2671058.
- ↑ Prasad A (February 1985). "Efficacy of trazodone as an anti obsessional agent". Pharmacol. Biochem. Behav. 22 (2): 347–8. doi:10.1016/0091-3057(85)90403-4. PMID 3983224.
- 1 2 Pigott TA, L'Heureux F, Rubenstein CS, Bernstein SE, Hill JL, Murphy DL; l'Heureux; Rubenstein; Bernstein; Hill; Murphy (June 1992). "A double-blind, placebo controlled study of trazodone in patients with obsessive-compulsive disorder". J Clin Psychopharmacol 12 (3): 156–62. doi:10.1097/00004714-199202000-00003. PMID 1629380.
- ↑ Roccatagliata G; Albano C; Maffini M; Farelli S (1980). "Alcohol withdrawal syndrome: treatment with trazodone". Int Pharmacopsychiatry. 15 (2): 105–10. PMID 6108298.
- ↑ Le Bon O; Murphy JR; Staner L; Hoffmann G; Kormoss N; Kentos M; Dupont P; Lion K; Pelc I; Verbanck P (August 2003). "Double-blind, placebo-controlled study of the efficacy of trazodone in alcohol post-withdrawal syndrome: polysomnographic and clinical evaluations". J Clin Psychopharmacol 23 (4): 377–83. doi:10.1097/01.jcp.0000085411.08426.d3. PMID 12920414.
- ↑ Borras L; de Timary P; Constant EL; Huguelet P; Eytan A (November 2006). "Successful treatment of alcohol withdrawal with trazodone". Pharmacopsychiatry 39 (6): 232. doi:10.1055/s-2006-951385. PMID 17124647.
- ↑ Singh, SP; Singh, V; Kar, N; Chan, K (2010). "Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis" (PDF). The British Journal of Psychiatry 197 (3): 174–179. doi:10.1192/bjp.bp.109.067710. PMID 20807960.
- ↑ Fink, HA; MacDonald, R; Rutks, IR; Wilt, TJ (September 2003). "Trazodone for erectile dysfunction: a systematic review and meta-analysis.". BJU International 92 (4): 441–446. doi:10.1046/j.1464-410X.2003.04358.x. PMID 12930437.
- ↑ Wesnes, KA; Simpson, PM; Christmas, L; Anand, R; McClelland, GR (1989). "The effects of moclobemide on cognition". J. Neural Transm. Suppl. 28: 91–102. PMID 2677245.
- 1 2 Schatzberg, AF; Nemeroff, CB, eds. (2009). Textbook of Psychopharmacology (4th ed.). Washington, D.C.: American Psychiatric Publishing. ISBN 978-1-58562-309-9.
- ↑ Abber, RE;; et al. (1987). "Priapism induced by chlorpromazine and trazodone: mechanism of action". J. Urol 137 (5): 1039–1042. PMID 3573170.
- ↑ National Institutes of Health. "Trazodone Side Effects -MedlinePlus". U.S. National Library of Medicine. National Institutes of Health. Retrieved 23 May 2011.
- ↑ Battaglia; ven Turoli RE (2009). "Persistent genital arousal disorder and trazodone. 5555Morphometric and vascular modifications of the clitoris. A case report". J Sex Med 6 (10): 2896–2900. doi:10.1111/j.1743-6109.2009.01418.x. PMID 19674253.
- ↑ Kalgutkar AS, Henne KR, Lame ME; Henne; Lame; Vaz; Collin; Soglia; Zhao; Hop (June 2005). "Metabolic activation of the nontricyclic antidepressant trazodone to electrophilic quinone-imine and epoxide intermediates in human liver microsomes and recombinant P4503A4". Chem Biol Interact. 155 (1–2): 10–20. doi:10.1016/j.cbi.2005.03.036. PMID 15978881.
- ↑ Otani K, Yasui N, Kaneko S; Yasui; Kaneko; Ishida; Ohkubo; Osanai; Sugawara; Fukushima (June 1995). "Trazodone treatment increases plasma prolactin concentrations in depressed patients". Int Clin Psychopharmacol 10 (2): 115–7. doi:10.1097/00004850-199506000-00009. PMID 7673654.
- ↑ "Webmd.com". Webmd.com. Retrieved 2014-03-14.
- 1 2 Rotzinger S, Fang J, Baker GB; Fang; Baker (1 June 1998). "Trazodone is metabolized to m-chlorophenylpiperazine by CYP3A4 from human sources". Drug Metab. Dispos. 26 (6): 572–5. PMID 9616194.
- ↑ Warner CH, Bobo W, Warner C, Reid S, Rachal J; Bobo; Warner; Reid; Rachal (August 2006). "Antidepressant discontinuation syndrome". Am Fam Physician 74 (3): 449–56. PMID 16913164.
- ↑ Einarson, A; Bonari, L; Voyer-Lavigne, S; Addis, A; Matsui, D; Johnson, Y; Koren, G (March 2003). "A multicentre prospective controlled study to determine the safety of trazodone and nefazodone use during pregnancy". Can J Psychiatry 48 (2): 106–110. PMID 12655908.
- ↑ Verbeeck, RK; Ross, SG; McKenna, EA (September 1986). "Excretion of trazodone in breast milk". Br J Clin Pharmacol 22 (3): 367–370. doi:10.1111/j.1365-2125.1986.tb02903.x. PMID 3768252.
- ↑ "Selective Serotonin Reuptake Inhibitor Toxicity". 2014-02-26.
- ↑ "Selective Serotonin Reuptake Inhibitor Toxicity". 2014-02-26.
- ↑ Martínez MA, Ballesteros S, Sánchez de la Torre C, Almarza E; Ballesteros; Sánchez de la Torre C; Almarza (2005). "Investigation of a fatality due to trazodone poisoning: case report and literature review". J Anal Toxicol 29 (4): 262–8. doi:10.1093/jat/29.4.262. PMID 15975258.
- ↑ de Meester A, Carbutti G, Gabriel L, Jacques JM; Carbutti; Gabriel; Jacques (2001). "Fatal overdose with trazodone: case report and literature review". Acta Clin Belg 56 (4): 258–61. doi:10.1179/acb.2001.038. PMID 11603256.
- ↑ Rakel RE (1987). "The greater safety of trazodone over tricyclic antidepressant agents: 5-year experience in the United States". Psychopathology 20 (Suppl 1): 57–63. doi:10.1159/000284524. PMID 3321131.
- ↑ Richelson E, Nelson A; Nelson (July 1984). "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". The Journal of Pharmacology and Experimental Therapeutics 230 (1): 94–102. PMID 6086881.
- ↑ Wander TJ, Nelson A, Okazaki H, Richelson E; Nelson; Okazaki; Richelson (December 1986). "Antagonism by antidepressants of serotonin S1 and S2 receptors of normal human brain in vitro". European Journal of Pharmacology 132 (2–3): 115–21. doi:10.1016/0014-2999(86)90596-0. PMID 3816971.
- ↑ Cusack B, Nelson A, Richelson E; Nelson; Richelson (May 1994). "Binding of antidepressants to human brain receptors: focus on newer generation compounds". Psychopharmacology 114 (4): 559–65. doi:10.1007/BF02244985. PMID 7855217.
- ↑ Pälvimäki EP, Roth BL, Majasuo H; et al. (August 1996). "Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor". Psychopharmacology 126 (3): 234–40. doi:10.1007/BF02246453. PMID 8876023.
- 1 2 Raffa RB, Shank RP, Vaught JL; Shank; Vaught (1992). "Etoperidone, trazodone and MCPP: in vitro and in vivo identification of serotonin 5-HT1A (antagonistic) activity". Psychopharmacology 108 (3): 320–6. doi:10.1007/BF02245118. PMID 1387963.
- 1 2 Odagaki Y, Toyoshima R, Yamauchi T; Toyoshima; Yamauchi (May 2005). "Trazodone and its active metabolite m-chlorophenylpiperazine as partial agonists at 5-HT1A receptors assessed by [35S]GTPgammaS binding". Journal of Psychopharmacology (Oxford, England) 19 (3): 235–41. doi:10.1177/0269881105051526. PMID 15888508.
- ↑ Tatsumi M, Groshan K, Blakely RD, Richelson E; Groshan; Blakely; Richelson (December 1997). "Pharmacological profile of antidepressants and related compounds at human monoamine transporters". European Journal of Pharmacology 340 (2–3): 249–58. doi:10.1016/S0014-2999(97)01393-9. PMID 9537821.
- ↑ Roth, BL; Driscol, J (2011-01-12). "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health.
- 1 2 3 Marek GJ, McDougle CJ, Price LH, Seiden LS; McDougle; Price; Seiden (1992). "A comparison of trazodone and fluoxetine: implications for a serotonergic mechanism of antidepressant action". Psychopharmacology 109 (1–2): 2–11. doi:10.1007/BF02245475. PMID 1365657.
- ↑ Vanina Y, Podolskaya A, Sedky K; et al. (July 2002). "Body weight changes associated with psychopharmacology". Psychiatric Services (Washington, D.C.) 53 (7): 842–7. doi:10.1176/appi.ps.53.7.842. PMID 12096167.
- ↑ Watanabe N, Omori IM, Nakagawa A; et al. (January 2010). "Safety reporting and adverse-event profile of mirtazapine described in randomized controlled trials in comparison with other classes of antidepressants in the acute-phase treatment of adults with depression: systematic review and meta-analysis". CNS Drugs 24 (1): 35–53. doi:10.2165/11319480-000000000-00000. PMID 20030418.
- ↑ Kinney GG, Griffith JC, Hudzik TJ; Griffith; Hudzik (July 1998). "Antidepressant-like effects of 5-hydroxytryptamine1A receptor agonists on operant responding under a response duration differentiation schedule". Behavioural Pharmacology 9 (4): 309–18. doi:10.1097/00008877-199807000-00002. PMID 10065919.
- ↑ Stahl, S.M. (2013). Stahl’s Essential Psychopharmacology (4th ed.). Cambridge University Press. ISBN 1107686466.
- ↑ Asayesh K (December 1986). "Combination of trazodone and phenothiazines: a possible additive hypotensive effect". Canadian Journal of Psychiatry 31 (9): 857–8. PMID 3802006.
- ↑ Melzacka M, Rurak, Vetulani (1980). "Preliminary study of the biotransformation of two new drugs, trazodone and etoperidone". Polish Journal of Pharmacology and Pharmacy 32 (4): 551–6. PMID 7255270.
- ↑ Fong MH, Garattini S, Caccia S; Garattini; Caccia (October 1982). "1-m-Chlorophenylpiperazine is an active metabolite common to the psychotropic drugs trazodone, etoperidone and mepiprazole". The Journal of Pharmacy and Pharmacology 34 (10): 674–5. doi:10.1111/j.2042-7158.1982.tb04701.x. PMID 6128394.
- ↑ Maes M, Westenberg H, Vandoolaeghe E; et al. (October 1997). "Effects of trazodone and fluoxetine in the treatment of major depression: therapeutic pharmacokinetic and pharmacodynamic interactions through formation of meta-chlorophenylpiperazine". Journal of Clinical Psychopharmacology 17 (5): 358–64. doi:10.1097/00004714-199710000-00004. PMID 9315986.
- ↑ Mihara K, Yasui-Furukori N, Kondo T; et al. (August 2002). "Relationship between plasma concentrations of trazodone and its active metabolite, m-chlorophenylpiperazine, and its clinical effect in depressed patients". Therapeutic Drug Monitoring 24 (4): 563–6. doi:10.1097/00007691-200208000-00016. PMID 12142643.
- ↑ Li AA, Marek GJ, Hand TH, Seiden LS; Marek; Hand; Seiden (February 1990). "Antidepressant-like effects of trazodone on a behavioral screen are mediated by trazodone, not the metabolite m-chlorophenylpiperazine". European Journal of Pharmacology 177 (3): 137–44. doi:10.1016/0014-2999(90)90263-6. PMID 2311675.
- ↑ Vetulani J, Sansone M, Baran L, Hano J; Sansone; Baran; Hano (1984). "Opposite action of m-chlorophenylpiperazine on avoidance depression induced by trazodone and pimozide in CD-1 mice". Psychopharmacology 83 (2): 166–8. doi:10.1007/BF00429728. PMID 6431467.
- ↑ Kast RE (2009). "Trazodone generates m-CPP: in 2008 risks from m-CPP might outweigh benefits of trazodone". World Journal of Biological Psychiatry 10 (4 Pt 2): 682–5. doi:10.1080/15622970902836022. PMID 19384678.
- ↑ Workman EA, Tellian F, Short D; Tellian; Short (May 1992). "Trazodone induction of migraine headache through mCPP". The American Journal of Psychiatry 149 (5): 712. doi:10.1176/ajp.149.5.712b. PMID 1575270.
- ↑ Jauch R, Kopitar Z, Prox A, Zimmer A; Kopitar; Prox; Zimmer (1976). "[Pharmacokinetics and metabolism of trazodone in man (author's transl)]" [Pharmacokinetics and metabolism of trazodone in man (author's transl)]. Arzneimittelforschung (in German) 26 (11): 2084–9. PMID 1037253.
- ↑ Logan BK, Costantino AG, Rieders EF, Sanders D; Costantino; Rieders; Sanders (Nov 2010). "Trazodone, meta-chlorophenylpiperazine (a hallucinogenic drug and trazodone metabolite), and the hallucinogen trifluoromethylphenylpiperazine cross-react with the EMIT®II ecstasy immunoassay in urine". J Anal Toxicol 34 (9): 587–9. doi:10.1093/jat/34.9.587. PMID 21073812.
- ↑ Brand NamesDesyrel, Oleptro. "Trazodone". rxwiki.com. Retrieved 2014-03-14.
- ↑ Silvestrini B (1989). "Trazodone: from the mental pain to the "dys-stress" hypothesis of depression". Clin Neuropharmacol 12 (Suppl 1): S4–10. doi:10.1097/00002826-198901001-00002. PMID 2568177.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||