Density functional theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (principally the ground state) of many-body systems, in particular atoms, molecules, and the condensed phases. Using this theory, the properties of a many-electron system can be determined by using functionals, i.e. functions of another function, which in this case is the spatially dependent electron density. Hence the name density functional theory comes from the use of functionals of the electron density. DFT is among the most popular and versatile methods available in condensed-matter physics, computational physics, and computational chemistry.
DFT has been very popular for calculations in solid-state physics since the 1970s. However, DFT was not considered accurate enough for calculations in quantum chemistry until the 1990s, when the approximations used in the theory were greatly refined to better model the exchange and correlation interactions. In many cases the results of DFT calculations for solid-state systems agree quite satisfactorily with experimental data. Computational costs are relatively low when compared to traditional methods, such as Hartree–Fock theory and its descendants based on the complex many-electron wavefunction.
Despite recent improvements, there are still difficulties in using density functional theory to properly describe intermolecular interactions (of critical importance to understanding chemical reactions), especially van der Waals forces (dispersion); charge transfer excitations; transition states, global potential energy surfaces, dopant interactions and some other strongly correlated systems; and in calculations of the band gap and ferromagnetism in semiconductors.[1] Its incomplete treatment of dispersion can adversely affect the accuracy of DFT (at least when used alone and uncorrected) in the treatment of systems which are dominated by dispersion (e.g. interacting noble gas atoms)[2] or where dispersion competes significantly with other effects (e.g. in biomolecules).[3] The development of new DFT methods designed to overcome this problem, by alterations to the functional and inclusion of additional terms to account for both core and valence electrons [4] or by the inclusion of additive terms,[5][6][7][8] is a current research topic.
Overview of method
Although density functional theory has its conceptual roots in the Thomas–Fermi model, DFT was put on a firm theoretical footing by the two Hohenberg–Kohn theorems (H–K).[9] The original H–K theorems held only for non-degenerate ground states in the absence of a magnetic field, although they have since been generalized to encompass these.[10][11]
The first H–K theorem demonstrates that the ground state properties of a many-electron system are uniquely determined by an electron density that depends on only 3 spatial coordinates. It lays the groundwork for reducing the many-body problem of N electrons with 3N spatial coordinates to 3 spatial coordinates, through the use of functionals of the electron density. This theorem can be extended to the time-dependent domain to develop time-dependent density functional theory (TDDFT), which can be used to describe excited states.
The second H–K theorem defines an energy functional for the system and proves that the correct ground state electron density minimizes this energy functional.
Within the framework of Kohn–Sham DFT (KS DFT), the intractable many-body problem of interacting electrons in a static external potential is reduced to a tractable problem of non-interacting electrons moving in an effective potential. The effective potential includes the external potential and the effects of the Coulomb interactions between the electrons, e.g., the exchange and correlation interactions. Modeling the latter two interactions becomes the difficulty within KS DFT. The simplest approximation is the local-density approximation (LDA), which is based upon exact exchange energy for a uniform electron gas, which can be obtained from the Thomas–Fermi model, and from fits to the correlation energy for a uniform electron gas. Non-interacting systems are relatively easy to solve as the wavefunction can be represented as a Slater determinant of orbitals. Further, the kinetic energy functional of such a system is known exactly. The exchange-correlation part of the total-energy functional remains unknown and must be approximated.
Another approach, less popular than KS DFT but arguably more closely related to the spirit of the original H-K theorems, is orbital-free density functional theory (OFDFT), in which approximate functionals are also used for the kinetic energy of the non-interacting system.
Derivation and formalism
As usual in many-body electronic structure calculations, the nuclei of the treated molecules or clusters are seen as fixed (the Born–Oppenheimer approximation), generating a static external potential V in which the electrons are moving. A stationary electronic state is then described by a wavefunction 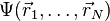 satisfying the many-electron time-independent Schrödinger equation
satisfying the many-electron time-independent Schrödinger equation
where, for the  -electron system,
-electron system, 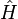 is the Hamiltonian,
is the Hamiltonian,  is the total energy,
is the total energy, 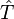 is the kinetic energy,
is the kinetic energy, 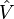 is the potential energy from the external field due to positively charged nuclei, and
is the potential energy from the external field due to positively charged nuclei, and  is the electron-electron interaction energy. The operators
is the electron-electron interaction energy. The operators 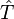 and
and  are called universal operators as they are the same for any
are called universal operators as they are the same for any  -electron system, while
-electron system, while 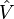 is
system dependent. This complicated many-particle equation is not separable into simpler single-particle equations because of the interaction term
is
system dependent. This complicated many-particle equation is not separable into simpler single-particle equations because of the interaction term  .
.
There are many sophisticated methods for solving the many-body Schrödinger equation based on the expansion of the wavefunction in Slater determinants. While the simplest one is the Hartree–Fock method, more sophisticated approaches are usually categorized as post-Hartree–Fock methods. However, the problem with these methods is the huge computational effort, which makes it virtually impossible to apply them efficiently to larger, more complex systems.
Here DFT provides an appealing alternative, being much more versatile as it provides a way to systematically map the many-body problem, with  , onto a single-body problem without
, onto a single-body problem without  . In DFT the key variable is the particle density
. In DFT the key variable is the particle density 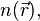 which for a normalized
which for a normalized 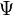 is given by
is given by
This relation can be reversed, i.e., for a given ground-state density 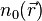 it is possible, in principle, to calculate the corresponding ground-state wavefunction
it is possible, in principle, to calculate the corresponding ground-state wavefunction 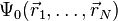 . In other words,
. In other words, 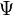 is a unique functional of
is a unique functional of  ,[9]
,[9]
and consequently the ground-state expectation value of an observable 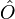 is also a functional of
is also a functional of 
In particular, the ground-state energy is a functional of 
where the contribution of the external potential ![\left\langle \Psi[n_0] \left|\hat V \right| \Psi[n_0] \right\rangle](../I/m/cd0cd966517148fb95c7c4d15bd1e44f.png) can be written explicitly in terms of the ground-state density
can be written explicitly in terms of the ground-state density 
More generally, the contribution of the external potential 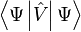 can be written explicitly in terms of the density
can be written explicitly in terms of the density  ,
,
The functionals ![\,\!T[n]](../I/m/2220bca035dc1ca4b3c43a0465cf7de4.png) and
and ![\,\!U[n]](../I/m/8c380361593608570ffd1e2013557443.png) are called universal functionals, while
are called universal functionals, while ![\,\!V[n]](../I/m/b40fbd957f5970efeed776fa2de69c73.png) is called a non-universal functional, as it depends on the system under study. Having specified a system, i.e., having specified
is called a non-universal functional, as it depends on the system under study. Having specified a system, i.e., having specified 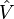 , one then has to minimize the functional
, one then has to minimize the functional
with respect to 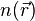 , assuming one has got reliable expressions for
, assuming one has got reliable expressions for ![\,\!T[n]](../I/m/2220bca035dc1ca4b3c43a0465cf7de4.png) and
and ![\,\!U[n]](../I/m/8c380361593608570ffd1e2013557443.png) . A successful minimization of the energy functional will yield the ground-state density
. A successful minimization of the energy functional will yield the ground-state density  and thus all other ground-state observables.
and thus all other ground-state observables.
The variational problems of minimizing the energy functional ![\,\!E[n]](../I/m/51999f8192d36af3a519560759a0ef40.png) can be solved by applying the Lagrangian method of undetermined multipliers.[12] First, one considers an energy functional that doesn't explicitly have an electron-electron interaction energy term,
can be solved by applying the Lagrangian method of undetermined multipliers.[12] First, one considers an energy functional that doesn't explicitly have an electron-electron interaction energy term,
where 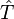 denotes the kinetic energy operator and
denotes the kinetic energy operator and 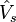 is an external effective potential in which the particles are moving, so that
is an external effective potential in which the particles are moving, so that 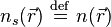 .
.
Thus, one can solve the so-called Kohn–Sham equations of this auxiliary non-interacting system,
which yields the orbitals 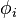 that reproduce the density
that reproduce the density 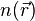 of the original many-body system
of the original many-body system
The effective single-particle potential can be written in more detail as
where the second term denotes the so-called Hartree term describing the electron-electron Coulomb repulsion, while the last term 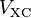 is called the exchange-correlation potential. Here,
is called the exchange-correlation potential. Here, 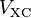 includes all the many-particle interactions. Since the Hartree term and
includes all the many-particle interactions. Since the Hartree term and 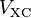 depend on
depend on 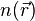 , which
depends on the
, which
depends on the 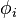 , which in turn depend on
, which in turn depend on 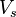 , the problem of solving the Kohn–Sham equation has to be done in a self-consistent (i.e., iterative) way. Usually one starts with an initial guess for
, the problem of solving the Kohn–Sham equation has to be done in a self-consistent (i.e., iterative) way. Usually one starts with an initial guess for 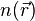 , then calculates the corresponding
, then calculates the corresponding 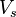 and solves the Kohn–Sham equations for the
and solves the Kohn–Sham equations for the 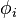 . From these one calculates a new density and starts again. This procedure is then repeated until convergence is reached. A non-iterative approximate formulation called Harris functional DFT is an alternative approach to this.
. From these one calculates a new density and starts again. This procedure is then repeated until convergence is reached. A non-iterative approximate formulation called Harris functional DFT is an alternative approach to this.
NOTE1: The one-to-one correspondence between electron density and single-particle potential is not so smooth. It contains kinds of non-analytic structure. ![E_s[n]](../I/m/57adbc773a1e2e86dadbd8f8b4bb534e.png) contains kinds of singularities, cuts and branches. This may indicate a limitation of our hope for representing exchange-correlation functional in a simple analytic form.
contains kinds of singularities, cuts and branches. This may indicate a limitation of our hope for representing exchange-correlation functional in a simple analytic form.
NOTE2: It is possible to extend the DFT idea to the case of Green function  instead of the density
instead of the density  . It is called as Luttinger-Ward functional (or kinds of similar functionals), written as
. It is called as Luttinger-Ward functional (or kinds of similar functionals), written as ![E[G]](../I/m/dacaaff7d8dcb4bfa5baf5af482122fe.png) . However,
. However, is determined not as its minimum, but as its extremum. Thus we may have some theoretical and practical difficulties.
is determined not as its minimum, but as its extremum. Thus we may have some theoretical and practical difficulties.
NOTE3: There is no one-to-one correspondence between one-body density matrix 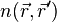 and
the one-body potential
and
the one-body potential 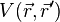 . (Remember that all the eigenvalues of
. (Remember that all the eigenvalues of 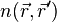 is unity). In other words, it ends up with a theory similar as the Hartree-Fock (or hybrid) theory.
is unity). In other words, it ends up with a theory similar as the Hartree-Fock (or hybrid) theory.
Approximations (exchange-correlation functionals)
The major problem with DFT is that the exact functionals for exchange and correlation are not known except for the free electron gas. However, approximations exist which permit the calculation of certain physical quantities quite accurately.[13] In physics the most widely used approximation is the local-density approximation (LDA), where the functional depends only on the density at the coordinate where the functional is evaluated:
The local spin-density approximation (LSDA) is a straightforward generalization of the LDA to include electron spin:
Highly accurate formulae for the exchange-correlation energy density
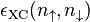 have been constructed
from quantum Monte Carlo simulations of jellium.[14]
have been constructed
from quantum Monte Carlo simulations of jellium.[14]
Generalized gradient approximations[15][16][17] (GGA) are still local but also take into account the gradient of the density at the same coordinate:
Using the latter (GGA) very good results for molecular geometries and ground-state energies have been achieved.
Potentially more accurate than the GGA functionals are the meta-GGA functionals, a natural development after the GGA (generalized gradient approximation). Meta-GGA DFT functional in its original form includes the second derivative of the electron density (the Laplacian) whereas GGA includes only the density and its first derivative in the exchange-correlation potential.
Functionals of this type are, for example, TPSS and the Minnesota Functionals. These functionals include a further term in the expansion, depending on the density, the gradient of the density and the Laplacian (second derivative) of the density.
Difficulties in expressing the exchange part of the energy can be relieved by including a component of the exact exchange energy calculated from Hartree–Fock theory. Functionals of this type are known as hybrid functionals.
Generalizations to include magnetic fields
The DFT formalism described above breaks down, to various degrees, in the presence of a vector potential, i.e. a magnetic field. In such a situation, the one-to-one mapping between the ground-state electron density and wavefunction is lost. Generalizations to include the effects of magnetic fields have led to two different theories: current density functional theory (CDFT) and magnetic field density functional theory (BDFT). In both these theories, the functional used for the exchange and correlation must be generalized to include more than just the electron density. In current density functional theory, developed by Vignale and Rasolt,[11] the functionals become dependent on both the electron density and the paramagnetic current density. In magnetic field density functional theory, developed by Salsbury, Grayce and Harris,[18] the functionals depend on the electron density and the magnetic field, and the functional form can depend on the form of the magnetic field. In both of these theories it has been difficult to develop functionals beyond their equivalent to LDA, which are also readily implementable computationally. Recently an extension by Pan and Sahni [19] extended the Hohenberg-Kohn theorem for non constant magnetic fields using the density and the current density as fundamental variables.
Applications

In general, density functional theory finds increasingly broad application in the chemical and material sciences for the interpretation and prediction of complex system behavior at an atomic scale. Specifically, DFT computational methods are applied for the study of systems to synthesis and processing parameters. In such systems, experimental studies are often encumbered by inconsistent results and non-equilibrium conditions. Examples of contemporary DFT applications include studying the effects of dopants on phase transformation behavior in oxides, magnetic behaviour in dilute magnetic semiconductor materials and the study of magnetic and electronic behavior in ferroelectrics and dilute magnetic semiconductors.[20][21] Also , it has been showed that DFT has a good results in the prediction of sensitivity of some nanostructures to environment pollutants like SO2 [22] or Acrolein [23]
In practice, Kohn–Sham theory can be applied in several distinct ways depending on what is being investigated. In solid state calculations, the local density approximations are still commonly used along with plane wave basis sets, as an electron gas approach is more appropriate for electrons delocalised through an infinite solid. In molecular calculations, however, more sophisticated functionals are needed, and a huge variety of exchange-correlation functionals have been developed for chemical applications. Some of these are inconsistent with the uniform electron gas approximation, however, they must reduce to LDA in the electron gas limit. Among physicists, probably the most widely used functional is the revised Perdew–Burke–Ernzerhof exchange model (a direct generalized-gradient parametrization of the free electron gas with no free parameters); however, this is not sufficiently calorimetrically accurate for gas-phase molecular calculations. In the chemistry community, one popular functional is known as BLYP (from the name Becke for the exchange part and Lee, Yang and Parr for the correlation part). Even more widely used is B3LYP which is a hybrid functional in which the exchange energy, in this case from Becke's exchange functional, is combined with the exact energy from Hartree–Fock theory. Along with the component exchange and correlation funсtionals, three parameters define the hybrid functional, specifying how much of the exact exchange is mixed in. The adjustable parameters in hybrid functionals are generally fitted to a 'training set' of molecules. Unfortunately, although the results obtained with these functionals are usually sufficiently accurate for most applications, there is no systematic way of improving them (in contrast to some of the traditional wavefunction-based methods like configuration interaction or coupled cluster theory). Hence in the current DFT approach it is not possible to estimate the error of the calculations without comparing them to other methods or experiments.
Thomas–Fermi model
The predecessor to density functional theory was the Thomas–Fermi model, developed independently by both Thomas and Fermi in 1927. They used a statistical model to approximate the distribution of electrons in an atom. The mathematical basis postulated that electrons are distributed uniformly in phase space with two electrons in every 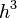 of volume.[24] For each element of coordinate space volume
of volume.[24] For each element of coordinate space volume 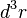 we can fill out a sphere of momentum space up to the Fermi momentum
we can fill out a sphere of momentum space up to the Fermi momentum 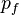 [25]
[25]
Equating the number of electrons in coordinate space to that in phase space gives:
Solving for  and substituting into the classical kinetic energy formula then leads directly to a kinetic energy represented as a functional of the electron density:
and substituting into the classical kinetic energy formula then leads directly to a kinetic energy represented as a functional of the electron density:
- where

As such, they were able to calculate the energy of an atom using this kinetic energy functional combined with the classical expressions for the nuclear-electron and electron-electron interactions (which can both also be represented in terms of the electron density).
Although this was an important first step, the Thomas–Fermi equation's accuracy is limited because the resulting kinetic energy functional is only approximate, and because the method does not attempt to represent the exchange energy of an atom as a conclusion of the Pauli principle. An exchange energy functional was added by Dirac in 1928.
However, the Thomas–Fermi–Dirac theory remained rather inaccurate for most applications. The largest source of error was in the representation of the kinetic energy, followed by the errors in the exchange energy, and due to the complete neglect of electron correlation.
Teller (1962) showed that Thomas–Fermi theory cannot describe molecular bonding. This can be overcome by improving the kinetic energy functional.
The kinetic energy functional can be improved by adding the Weizsäcker (1935) correction:[26][27]
Hohenberg–Kohn theorems
1.If two systems of electrons, one trapped in a potential 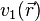 and the other in
and the other in 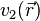 , have the same ground-state density
, have the same ground-state density 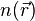 then necessarily
then necessarily  .
.
Corollary: the ground state density uniquely determines the potential and thus all properties of the system, including the many-body wave function. In particular, the "HK" functional, defined as ![F[n]=T[n]+U[n]](../I/m/0ae181635a28fd79322ece8255934552.png) is a universal functional of the density (not depending explicitly on the external potential).
is a universal functional of the density (not depending explicitly on the external potential).
2. For any positive integer 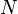 and potential
and potential 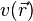 , a density functional
, a density functional ![F[n]](../I/m/f1ed56e4bc0fe1641e3e07c6a6b82c6c.png) exists such that
exists such that ![E_{(v,N)}[n] = F[n]+\int{v(\vec r)n(\vec r)d^3r}](../I/m/0bd9c16478db3e66a4ce1c7ff671124b.png) obtains its minimal value at the ground-state density of
obtains its minimal value at the ground-state density of 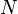 electrons in the potential
electrons in the potential 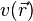 . The minimal value of
. The minimal value of ![E_{(v,N)}[n]](../I/m/2981e45300ed4aee332dbdf634e45303.png) is then the ground state energy of this system.
is then the ground state energy of this system.
Pseudo-potentials
The many electron Schrödinger equation can be very much simplified if electrons are divided in two groups: valence electrons and inner core electrons. The electrons in the inner shells are strongly bound and do not play a significant role in the chemical binding of atoms; they also partially screen the nucleus, thus forming with the nucleus an almost inert core. Binding properties are almost completely due to the valence electrons, especially in metals and semiconductors. This separation suggests that inner electrons can be ignored in a large number of cases, thereby reducing the atom to an ionic core that interacts with the valence electrons. The use of an effective interaction, a pseudopotential, that approximates the potential felt by the valence electrons, was first proposed by Fermi in 1934 and Hellmann in 1935. In spite of the simplification pseudo-potentials introduce in calculations, they remained forgotten until the late 50's.
Ab initio Pseudo-potentials
A crucial step toward more realistic pseudo-potentials was given by Topp and Hopfield and more recently Cronin, who suggested that the pseudo-potential should be adjusted such that they describe the valence charge density accurately. Based on that idea, modern pseudo-potentials are obtained inverting the free atom Schrödinger equation for a given reference electronic configuration and forcing the pseudo wave-functions to coincide with the true valence wave functions beyond a certain distance 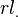 . The pseudo wave-functions are also forced to have the same norm as the true valence wave-functions and can be written as
. The pseudo wave-functions are also forced to have the same norm as the true valence wave-functions and can be written as
where 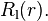 is the radial part of the wavefunction with angular momentum
is the radial part of the wavefunction with angular momentum 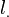 , and
, and 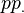 and
and 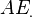 denote, respectively, the pseudo wave-function and the true (all-electron) wave-function. The index n in the true wave-functions denotes the valence level. The distance beyond which the true and the pseudo wave-functions are equal,
denote, respectively, the pseudo wave-function and the true (all-electron) wave-function. The index n in the true wave-functions denotes the valence level. The distance beyond which the true and the pseudo wave-functions are equal, 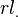 , is also
, is also 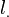 -dependent.
-dependent.
Software supporting DFT
DFT is supported by many Quantum chemistry and solid state physics software packages, often along with other methods.
See also
- Basis set (chemistry)
- Dynamical mean field theory
- Gas in a box
- Harris functional
- Helium atom
- Kohn–Sham equations
- Local density approximation
- Molecule
- Molecular design software
- Molecular modelling
- Quantum chemistry
- Thomas–Fermi model
- Time-dependent density functional theory
Lists
- List of quantum chemistry and solid state physics software
- List of software for molecular mechanics modeling
References
- ↑ Assadi, M.H.N; et al. (2013). "Theoretical study on copper's energetics and magnetism in TiO2 polymorphs" (PDF). Journal of Applied Physics 113 (23): 233913. arXiv:1304.1854. Bibcode:2013JAP...113w3913A. doi:10.1063/1.4811539.
- ↑ Van Mourik, Tanja; Gdanitz, Robert J. (2002). "A critical note on density functional theory studies on rare-gas dimers". Journal of Chemical Physics 116 (22): 9620–9623. Bibcode:2002JChPh.116.9620V. doi:10.1063/1.1476010.
- ↑ Vondrášek, Jiří; Bendová, Lada; Klusák, Vojtěch; Hobza, Pavel (2005). "Unexpectedly strong energy stabilization inside the hydrophobic core of small protein rubredoxin mediated by aromatic residues: correlated ab initio quantum chemical calculations". Journal of the American Chemical Society 127 (8): 2615–2619. doi:10.1021/ja044607h. PMID 15725017.
- ↑ Grimme, Stefan (2006). "Semiempirical hybrid density functional with perturbative second-order correlation". Journal of Chemical Physics 124 (3): 034108. Bibcode:2006JChPh.124c4108G. doi:10.1063/1.2148954. PMID 16438568.
- ↑ Zimmerli, Urs; Parrinello, Michele; Koumoutsakos, Petros (2004). "Dispersion corrections to density functionals for water aromatic interactions". Journal of Chemical Physics 120 (6): 2693–2699. Bibcode:2004JChPh.120.2693Z. doi:10.1063/1.1637034. PMID 15268413.
- ↑ Grimme, Stefan (2004). "Accurate description of van der Waals complexes by density functional theory including empirical corrections". Journal of Computational Chemistry 25 (12): 1463–1473. doi:10.1002/jcc.20078. PMID 15224390.
- ↑ Von Lilienfeld, O. Anatole; Tavernelli, Ivano; Rothlisberger, Ursula; Sebastiani, Daniel (2004). "Optimization of effective atom centered potentials for London dispersion forces in density functional theory". Physical Review Letters 93 (15): 153004. Bibcode:2004PhRvL..93o3004V. doi:10.1103/PhysRevLett.93.153004. PMID 15524874.
- ↑ Tkatchenko, Alexandre; Scheffler, Matthias (2009). "Accurate Molecular Van Der Waals Interactions from Ground-State Electron Density and Free-Atom Reference Data". Physical Review Letters 102 (7): 073005. Bibcode:2009PhRvL.102g3005T. doi:10.1103/PhysRevLett.102.073005. PMID 19257665.
- 1 2 Hohenberg, Pierre; Walter Kohn (1964). "Inhomogeneous electron gas". Physical Review 136 (3B): B864–B871. Bibcode:1964PhRv..136..864H. doi:10.1103/PhysRev.136.B864.
- ↑ Levy, Mel (1979). "Universal variational functionals of electron densities, first-order density matrices, and natural spin-orbitals and solution of the v-representability problem". Proceedings of the National Academy of Sciences (United States National Academy of Sciences) 76 (12): 6062–6065. Bibcode:1979PNAS...76.6062L. doi:10.1073/pnas.76.12.6062.
- 1 2 Vignale, G.; Mark Rasolt (1987). "Density-functional theory in strong magnetic fields". Physical Review Letters (American Physical Society) 59 (20): 2360–2363. Bibcode:1987PhRvL..59.2360V. doi:10.1103/PhysRevLett.59.2360. PMID 10035523.
- ↑ Kohn, W.; Sham, L. J. (1965). "Self-consistent equations including exchange and correlation effects". Physical Review 140 (4A): A1133–A1138. Bibcode:1965PhRv..140.1133K. doi:10.1103/PhysRev.140.A1133.
- ↑ Kieron Burke and Lucas O. Wagner (2013). "DFT in a nutshell". International Journal of Quantum Chemistry 113 (2): 96. doi:10.1002/qua.24259.
- ↑ John P. Perdew, Adrienn Ruzsinszky, Jianmin Tao, Viktor N. Staroverov, Gustavo Scuseria and Gábor I. Csonka (2005). "Prescriptions for the design and selection of density functional approximations: More constraint satisfaction with fewer fits". Journal of Chemical Physics 123 (6): 062201. Bibcode:2005JChPh.123f2201P. doi:10.1063/1.1904565. PMID 16122287.
- ↑ Perdew, John P; Chevary, J A; Vosko, S H; Jackson, Koblar, A; Pederson, Mark R; Singh, D J; Fiolhais, Carlos (1992). "Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation". Physical Review B 46 (11): 6671. Bibcode:1992PhRvB..46.6671P. doi:10.1103/physrevb.46.6671.
- ↑ Becke, Axel D (1988). "Density-functional exchange-energy approximation with correct asymptotic behavior". Physical Review A 38 (6): 3098. Bibcode:1988PhRvA..38.3098B. doi:10.1103/physreva.38.3098.
- ↑ Langreth, David C; Mehl, M J (1983). "Beyond the local-density approximation in calculations of ground-state electronic properties". Physical Review B 28 (4): 1809. Bibcode:1983PhRvB..28.1809L. doi:10.1103/physrevb.28.1809.
- ↑ Grayce, Christopher; Robert Harris (1994). "Magnetic-field density-functional theory". Physical Review A 50 (4): 3089–3095. Bibcode:1994PhRvA..50.3089G. doi:10.1103/PhysRevA.50.3089. PMID 9911249.
- ↑ Viraht, Xiao-Yin (2012). "Hohenberg-Kohn theorem including electron spin". Physical Review A 86. Bibcode:1994PhRvA.86.042502. doi:10.1103/physreva.86.042502.
- ↑ Segall, M.D.; Lindan, P.J (2002). "First-principles simulation: ideas, illustrations and the CASTEP code". Journal of Physics: Condensed Matter 14 (11): 2717. Bibcode:2002JPCM...14.2717S. doi:10.1088/0953-8984/14/11/301.
- ↑ "Ab initio study of phase stability in doped TiO2". Computational Mechanics 50 (2): 185–194. 2012. doi:10.1007/s00466-012-0728-4.
- ↑ Somayeh. F. Rastegar, Hamed Soleymanabadi (2014-01-01). "Theoretical investigation on the selective detection of SO2 molecule by AlN nanosheets". Journal of Molecular Modeling.
- ↑ Somayeh F. Rastegar, Hamed Soleymanabadi (2013-01-01). "DFT studies of acrolein molecule adsorption on pristine and Al- doped graphenes". Journal of Molecular Modeling.
- ↑ (Parr & Yang 1989, p. 47)
- ↑ March, N. H. (1992). Electron Density Theory of Atoms and Molecules. Academic Press. p. 24. ISBN 0-12-470525-1.
- ↑ Weizsäcker, C. F. v. (1935). "Zur Theorie der Kernmassen". Zeitschrift für Physik 96 (7–8): 431–58. Bibcode:1935ZPhy...96..431W. doi:10.1007/BF01337700.
- ↑ (Parr & Yang 1989, p. 127)
Key papers
- Parr, R. G.; Yang, W. (1989). Density-Functional Theory of Atoms and Molecules. New York: Oxford University Press. ISBN 0-19-504279-4. ISBN 0-19-509276-7 (paperback).
- Thomas, L. H. (1927). "The calculation of atomic fields". Proc. Camb. Phil. Soc 23 (5): 542–548. Bibcode:1927PCPS...23..542T. doi:10.1017/S0305004100011683.
- Hohenberg, P.; Kohn, W. (1964). "Inhomogeneous Electron Gas". Physical Review 136 (3B): B864. Bibcode:1964PhRv..136..864H. doi:10.1103/PhysRev.136.B864.
- Kohn, W.; Sham, L. J. (1965). "Self-Consistent Equations Including Exchange and Correlation Effects". Physical Review 140 (4A): A1133. Bibcode:1965PhRv..140.1133K. doi:10.1103/PhysRev.140.A1133.
- Becke, Axel D. (1993). "Density-functional thermochemistry. III. The role of exact exchange". The Journal of Chemical Physics 98 (7): 5648. Bibcode:1993JChPh..98.5648B. doi:10.1063/1.464913.
- Lee, Chengteh; Yang, Weitao; Parr, Robert G. (1988). "Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density". Physical Review B 37 (2): 785. Bibcode:1988PhRvB..37..785L. doi:10.1103/PhysRevB.37.785.
- Burke, Kieron; Werschnik, Jan; Gross, E. K. U. (2005). "Time-dependent density functional theory: Past, present, and future". The Journal of Chemical Physics 123 (6): 062206. arXiv:cond-mat/0410362. Bibcode:2005JChPh.123f2206B. doi:10.1063/1.1904586.
External links
- Walter Kohn, Nobel Laureate Freeview video interview with Walter on his work developing density functional theory by the Vega Science Trust.
- Klaus Capelle, A bird's-eye view of density-functional theory
- Walter Kohn, Nobel Lecture
- Density functional theory on arxiv.org
- FreeScience Library -> Density Functional Theory
- Density Functional Theory – an introduction
- Electron Density Functional Theory – Lecture Notes
- Density Functional Theory through Legendre Transformationpdf
- Kieron Burke : Book On DFT : " THE ABC OF DFT " http://dft.uci.edu/doc/g1.pdf
- Modeling Materials Continuum, Atomistic and Multiscale Techniques, Book
![\hat H \Psi = \left[{\hat T}+{\hat V}+{\hat U}\right]\Psi = \left[\sum_i^N \left(-\frac{\hbar^2}{2m_i}\nabla_i^2\right) + \sum_i^N V(\vec r_i) + \sum_{i<j}^N U(\vec r_i, \vec r_j)\right] \Psi = E \Psi](../I/m/d4b49872bfc95388ef4808d5c80d4f45.png)
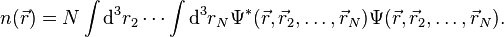
![\,\!\Psi_0 = \Psi[n_0]](../I/m/27347d299383b927bcef534699fa7035.png)
![O[n_0] = \left\langle \Psi[n_0] \left| \hat O \right| \Psi[n_0] \right\rangle.](../I/m/b00b36eba3a44a4ddf0b9542aabe4aa2.png)
![E_0 = E[n_0] = \left\langle \Psi[n_0] \left| \hat T + \hat V + \hat U \right| \Psi[n_0] \right\rangle](../I/m/c6482ef0878d54103ac0f04ede7479a0.png)
![V[n_0] = \int V(\vec r) n_0(\vec r){\rm d}^3r.](../I/m/b10977a3a93fb2d51d4c278a41373a0f.png)
![V[n] = \int V(\vec r) n(\vec r){\rm d}^3r.](../I/m/5795bfad63753d0007ec32019fc2ef18.png)
![E[n] = T[n]+ U[n] + \int V(\vec r) n(\vec r){\rm d}^3r](../I/m/a77376c23cf64fcf19999bb0f021d90f.png)
![E_s[n] = \left\langle \Psi_s[n] \left| \hat T + \hat V_s \right| \Psi_s[n] \right\rangle](../I/m/eeaccbdc873bf2fc6fe68f4f2171fb6a.png)
![\left[-\frac{\hbar^2}{2m}\nabla^2+V_s(\vec r)\right] \phi_i(\vec r) = \epsilon_i \phi_i(\vec r)](../I/m/fb393974bd442633dd64993db7ff7c80.png)
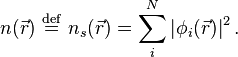
![V_s(\vec r) = V(\vec r) + \int \frac{e^2n_s(\vec r\,')}{|\vec r-\vec r\,'|} {\rm d}^3r' + V_{\rm XC}[n_s(\vec r)]](../I/m/bf220ad034de0ba662f84d2d106231eb.png)
![E_{\rm XC}^{\rm LDA}[n]=\int\epsilon_{\rm XC}(n)n (\vec{r}) {\rm d}^3r.](../I/m/dd70e3d29b2067cc09e9fcd2d7554fa1.png)
![E_{\rm XC}^{\rm LSDA}[n_\uparrow,n_\downarrow]=\int\epsilon_{\rm XC}(n_\uparrow,n_\downarrow)n (\vec{r}){\rm d}^3r.](../I/m/5cad4b1ec6680e049fffc540011c44e9.png)
![E_{XC}^{\rm GGA}[n_\uparrow,n_\downarrow]=\int\epsilon_{XC}(n_\uparrow,n_\downarrow,\vec{\nabla}n_\uparrow,\vec{\nabla}n_\downarrow)
n (\vec{r}) {\rm d}^3r.](../I/m/3546d451ea060a29fc9a160386ece7ab.png)
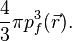
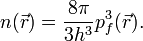
![t_{TF}[n] = \frac{p^2}{2m_e} \propto \frac{(n^\frac13)^2}{2m_e} \propto n^\frac23(\vec{r})](../I/m/dfdde4a4f1400daee9e3c01e02f85a26.png)
![T_{TF}[n]= C_F \int n(\vec{r}) n^\frac23(\vec{r}) d^3r =C_F\int n^\frac53(\vec{r}) d^3r](../I/m/93a368a740d917fcbeed0f341babd87d.png)
![T_W[n]=\frac{\hbar^2}{8m}\int\frac{|\nabla n(\vec{r})|^2}{n(\vec{r})}d^3r.](../I/m/9435727b9cbf5fd002d1a3ecf1b57b3f.png)