Denaturation (biochemistry)

Process of partial or total alteration of the native secondary, and/or tertiary, and/or quaternary structures of proteins or nucleic acids resulting in a loss of bioactivity.
Note 1: Modified from the definition given in ref.[1]
Note 2: Denaturation can occur when proteins and nucleic acids are subjected to elevated temperature or to extremes of pH, or to nonphysiological concentrations of salt, organic solvents, urea, or other chemical agents.
Note 3: An enzyme loses its catalytic activity when it is denaturized.[2]
Denaturation is a process in which proteins or nucleic acids lose the quaternary structure, tertiary structure and secondary structure which is present in their native state, by application of some external stress or compound such as a strong acid or base, a concentrated inorganic salt, an organic solvent (e.g., alcohol or chloroform), radiation or heat.[3] If proteins in a living cell are denatured, this results in disruption of cell activity and possibly cell death. Denatured proteins can exhibit a wide range of characteristics, from conformational change and loss of solubility to aggregation due to the exposure of hydrophobic groups.
Protein folding is key to whether a globular protein or a membrane protein can do its job correctly. It must be folded into the right shape to function. But hydrogen bonds, which play a big part in folding, are rather weak, and it doesn't take much heat, acidity, or other stress to break some and form others, denaturing the protein. This is one reason why tight homeostasis is physiologically necessary in many life forms.
This concept is unrelated to denatured alcohol, which is alcohol that has been mixed with additives to make it unsuitable for human consumption.
Common examples
When food is cooked, some of its proteins become denatured. This is why boiled eggs become hard and cooked meat becomes firm.
A classic example of denaturing in proteins comes from egg whites, which are typically largely egg albumins in water. Fresh from the eggs, egg whites are transparent and liquid. Cooking the thermally unstable whites turns them opaque, forming an interconnected solid mass. The same transformation can be effected with a denaturing chemical. Pouring egg whites into a beaker of acetone will also turn egg whites translucent and solid. The skin that forms on curdled milk is another common example of denatured protein. The cold appetizer known as ceviche is prepared by chemically "cooking" raw fish and shellfish in an acidic citrus marinade, without heat.[4]
Protein denaturation
Denatured proteins can exhibit a wide range of characteristics, from loss of solubility to protein aggregation."

1) Primary Structure : the linear structure of amino acids in the polypeptide chain
2) Secondary Structure : hydrogen bonds between peptide group chains in an alpha helix or beta
3) Tertiary Structure : three-dimensional structure of alpha helixes and beta helixes folded
4) Quaternary Structure : three-dimensional structure of multiple polypeptides and how they fit together
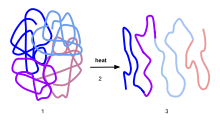
Background
Proteins are amino acid polymers. A protein is created by ribosomes that "read" RNA that is encoded by codons in the gene and assemble the requisite amino acid combination from the genetic instruction, in a process known as translation. The newly created protein strand then undergoes posttranslational modification, in which additional atoms or molecules are added, for example copper, zinc, or iron. Once this post-translational modification process has been completed, the protein begins to fold (sometimes spontaneously and sometimes with enzymatic assistance), curling up on itself so that hydrophobic elements of the protein are buried deep inside the structure and hydrophilic elements end up on the outside. The final shape of a protein determines how it interacts with its environment.
When a protein is denatured, secondary and tertiary structures are altered but the peptide bonds of the primary structure between the amino acids are left intact. Since all structural levels of the protein determine its function, the protein can no longer perform its function once it has been denatured. This is in contrast to intrinsically unstructured proteins, which are unfolded in their native state, but still functionally active.
How denaturation occurs at levels of protein structure
- In quaternary structure denaturation, protein sub-units are dissociated and/or the spatial arrangement of protein subunits is disrupted.
- Tertiary structure denaturation involves the disruption of:
- Covalent interactions between amino acid side-chains (such as disulfide bridges between cysteine groups)
- Non-covalent dipole-dipole interactions between polar amino acid side-chains (and the surrounding solvent)
- Van der Waals (induced dipole) interactions between nonpolar amino acid side-chains.
- In secondary structure denaturation, proteins lose all regular repeating patterns such as alpha-helices and beta-pleated sheets, and adopt a random coil configuration.
- Primary structure, such as the sequence of amino acids held together by covalent peptide bonds, is not disrupted by denaturation.[5]
Loss of function
Most biological substrates lose their biological function when denatured. For example, enzymes lose their activity, because the substrates can no longer bind to the active site, and because amino acid residues involved in stabilizing substrates' transition states are no longer positioned to be able to do so. The denaturing process and the associated loss of activity can be measured using techniques such as dual polarization interferometry, CD, and QCMD.
Reversibility and irreversibility
In very few cases, denaturation is reversible (the proteins can regain their native state when the denaturing influence is removed). This process can be called renaturation.[6] This understanding has led to the notion that all the information needed for proteins to assume their native state was encoded in the primary structure of the protein, and hence in the DNA that codes for the protein, the so-called "Anfinsen's thermodynamic hypothesis".[7] One example of renaturation is that an egg white can be uncooked using vitamin C or sodium borohydride.[8]
Nucleic acid denaturation
The denaturation of nucleic acids such as DNA due to high temperatures is the separation of a double strand into two single strands, which occurs when the hydrogen bonds between the strands are broken. This process is used during polymerase chain reaction. Nucleic acid strands realign when "normal" conditions are restored during annealing. If the conditions are restored too quickly, the nucleic acid strands may realign imperfectly.
Denaturants
Acids
Acidic protein denaturants include:
- Acetic acid[9]
- Trichloroacetic acid 12% in water
- Sulfosalicylic acid
Bases
Bases work similarly to acids in denaturation. They include:
Solvents
Most organic solvents are denaturing, including:
Cross-linking reagents
Cross-linking agents for proteins include:
Chaotropic agents
Chaotropic agents include:
- Urea 6 – 8 mol/l
- Guanidinium chloride 6 mol/l
- Lithium perchlorate 4.5 mol/l
Disulfide bond reducers
Agents that break disulfide bonds by reduction include:
- 2-Mercaptoethanol
- Dithiothreitol
- TCEP (tris(2-carboxyethyl)phosphine)
Other
- Mechanical agitation
- Picric acid
- Radiation
- Temperature[10]
See also
References
- ↑ Alan D. MacNaught, Andrew R. Wilkinson, ed. (1997). Compendium of Chemical Terminology: IUPAC Recommendations (the "Gold Book"). Blackwell Science. ISBN 0865426848.
- ↑ "Terminology for biorelated polymers and applications (IUPAC Recommendations 2012)" (PDF). Pure and Applied Chemistry 84 (2): 377–410. 2012. doi:10.1351/PAC-REC-10-12-04.
- ↑ Mosby's Medical Dictionary (8th ed.). Elsevier. 2009. Retrieved September 2013.
- ↑ "Ceviche: the new sushi," The Times.
- ↑ Charles Tanford (1968), "Protein denaturation" (PDF), Advances in Protein Chemistry 23: 121–282, doi:10.1016/S0065-3233(08)60401-5, PMID 4882248
- ↑ Campbell, N. A., Reece, J.B., Meyers, N., Urry, L. A., Cain, M.L., Wasserman, S.A., Minorsky, P.V., Jackson, R.B. (2009), Biology (8th, Australian version ed.), Sydney: Pearson Education Australia
- ↑ Anfinsen CB. (1973), "Principles that govern the folding of protein chains", Science 181 (4096): 223–30, doi:10.1126/science.181.4096.223, PMID 4124164
- ↑ http://www.neatorama.com/2008/02/26/herv-this-the-man-who-can-unboil-an-egg/#!EW0FX
- ↑ López-Alonso JP, Bruix M, Font J, Ribó M, Vilanova M, Jiménez MA, Santoro J, González C, Laurents DV. (2010), "NMR spectroscopy reveals that RNase A is chiefly denatured in 40% acetic acid: implications for oligomer formation by 3D domain swapping", J. Am. Chem. Soc. 132 (5): 1621–30, doi:10.1021/ja9081638, PMID 20085318
- ↑ Jaremko, M.; Jaremko Ł; Kim HY; Cho MK; Schwieters CD; Giller K; Becker S; Zweckstetter M. (April 2013). "Cold denaturation of a protein dimer monitored at atomic resolution". Nat. Chem. Biol. 9 (4): 264–70. doi:10.1038/nchembio.1181. PMID 23396077.