Chlorphenamine
 | |
| Systematic (IUPAC) name | |
|---|---|
|
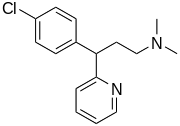
3-(4-chlorophenyl)-N,N-dimethyl- 3-pyridin-2-yl-propan-1-amine | |
| Clinical data | |
| Trade names | Chlor-trimeton |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a682543 |
| Pregnancy category | |
| Legal status |
|
| Routes of administration | Oral, IV, IM, SC |
| Pharmacokinetic data | |
| Bioavailability | 25 to 50% |
| Protein binding | 72% |
| Metabolism | Hepatic (CYP2D6) |
| Biological half-life | 21–27 hours |
| Excretion | Renal |
| Identifiers | |
| CAS Number |
113-92-8 |
| ATC code | R06AB04 |
| PubChem | CID 2725 |
| IUPHAR/BPS | 1210 |
| DrugBank |
DB01114 |
| ChemSpider |
2624 |
| UNII |
3U6IO1965U |
| KEGG |
D07398 |
| ChEBI |
CHEBI:52010 |
| ChEMBL |
CHEMBL505 |
| Chemical data | |
| Formula | C16H19ClN2 |
| Molar mass | 274.788 g/mol |
| |
| |
| Physical data | |
| Solubility in water | 0.55 g/100 mL, liquid mg/mL (20 °C) |
| | |
Chlorphenamine (INN), chlorpheniramine (USAN, former BAN) or piriton (trade name), commonly marketed in the form of chlorphenamine maleate (Chlorphen-12[1]), is a first-generation alkylamine antihistamine used in the prevention of the symptoms of allergic conditions such as rhinitis and urticaria. Its sedative effects are relatively weak compared to other first-generation antihistamines. Chlorphenamine is one of the most commonly used antihistamines in small-animal veterinary practice. Although not generally approved as an antidepressant or anti-anxiety medication, chlorphenamine appears to have these properties as well.[2][3]
Chlorphenamine is part of a series of antihistamines including pheniramine (Naphcon) and its halogenated derivatives and others including fluorpheniramine, dexchlorpheniramine (Polaramine), brompheniramine (Dimetapp), dexbrompheniramine (Drixoral), deschlorpheniramine, triprolidine (Actifed), and iodopheniramine.
The halogenated alkylamine antihistamines all exhibit optical isomerism, and chlorphenamine in the indicated products is racemic chlorphenamine maleate, whereas dexchlorpheniramine is the dextrorotary stereoisomer.
Serotonergic and norepinephrinergic effects
In addition to being an histamine H1 receptor (HRH1) antagonist, chlorphenamine has been shown to work as a serotonin-norepinephrine reuptake inhibitor or SNRI.[4] A similar antihistamine, brompheniramine, led to the discovery of the SSRI zimelidine. Limited clinical evidence shows that it is comparable to several antidepressant medications in its ability to inhibit the reuptake of serotonin and also norepinephrine (noradrenaline).[5]
A study performed on Fischer 344/Brown Norway F1 hybrid rats showed that intraventricular administration of chlorphenamine reduced fear-related behaviors and improved maze performance. It was also noted that long-term administration of chlorphenamine reduced age-related deficits in motor function.[6]
Combination medications
Chlorphenamine is often combined with phenylpropanolamine to form an allergy medication with both antihistamine and decongestant properties, though phenylpropanolamine is no longer available in the US after studies showed it increased the risk of stroke in young women. Chlorpheniramine remains available with no such risk. Brand names had included Demazin, Allerest 12 Hour, Codral Nighttime, Chlornade, Contac 12 Hour, Exchange Select Allergy Multi-Symptom, A. R. M. Allergy Relief, Ordrine, Ornade Spansules, Teldrin, Triaminic, and Tylenol Cold/Allergy.
Chlorphenamine is combined with a narcotic (hydrocodone) in the product Tussionex, which is indicated for treatment of cough and upper respiratory symptoms associated with allergy or cold in adults and children 6 years of age and older.[7] This combination is manufactured as a time-released formula, which allows for administration every 12 hours, versus the more common 4-to-6-hour regimen for other narcotic cough suppressants.
Chlorphenamine/dihydrocodeine immediate-release syrups are also marketed. The antihistamine is helpful in cases where allergy or common cold is the reason for the cough; it is also a potentiator of opioids, allowing enhanced suppression of cough, analgesia, and other effects from a given quantity of the drug by itself. In various places in the world, cough & cold preparations containing codeine and chlorphenamine are available.
In the drug Coricidin, chlorphenamine is combined with the cough suppressant dextromethorphan.
Adverse effects
The adverse effects include drowsiness, dizziness, confusion, constipation, anxiety, nausea, blurred vision, restlessness, decreased coordination, dry mouth, shallow breathing, hallucinations, irritability, problems with memory or concentration, tinnitus and trouble urinating.
A large study linked the development of Alzheimer's disease and other forms of dementia to the use of chlorpheniramine and other first-generation antihistamines, due to their anticholinergic properties.[8]
Synthesis
There are several patented methods for the synthesis of chlorphenamine. In one example, 4-chlorobenzylcyanide is reacted with 2-chloropyridine in the presence of sodium amide to form 4-chlorophenyl(2-pyridyl)acetonitrile. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives γ-(4-chlorphenyl)-γ-cyano-N,N-dimethyl-2-pyridinepropanamine, the hydrolysis and decarboxylation of which lead to chlorphenamine.

A second method starts from pyridine, which undergoes alkylation by 4-chlorobenzylchloride,[9] giving 2-(4-chlorobenzyl)pyridine. Alkylating this with 2-dimethylaminoethylchloride in the presence of sodium amide gives chlorphenamine.

See also
References
- ↑ "Chlorphen-12".
- ↑ Carlsson, Arvid; Lindqvist, Margit. "Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines". Journal of Pharmacy and Pharmacology. Journal of Pharmacy and Pharmacology. Retrieved 1 December 2013.
- ↑ Gruetter CA, Lemke SM, Anestis DK, Szarek JL, Valentovic MA. (Jul 1992). "Potentiation of 5-hydroxytryptamine-induced contraction in rat aorta by chlorpheniramine, citalopram and fluoxetine". Eur J Pharmacol (Department of Pharmacology, Marshall University School of Medicine, Huntington, WV) 217: 109–18. PMID 1358631. Retrieved 1 December 2013.
- ↑ Carlsson, A.; Linqvist M. (1969). "Central and peripheral monoaminergic membrane-pump blockade by some addictive analgesics and antihistamines". Journal of Pharmacy and Pharmacology 21 (7): 460–464. doi:10.1111/j.2042-7158.1969.tb08287.x. PMID 4390069.
- ↑ Hellbom, E. (2006). "Chlorpheniramine, selective serotonin-reuptake inhibitors (SSRIs) and over-the-counter (OTC) treatment". Medical Hypotheses 66 (4): 689–690. doi:10.1016/j.mehy.2005.12.006. PMID 16413139.
- ↑ Hasenöhrl, R. U.; Weth, K.; Huston, J. P. (1999). "Intraventricular infusion of the histamine H1 receptor antagonist chlorpheniramine improves maze performance and has anxiolytic-like effects in aged hybrid Fischer 344×Brown Norway rats". Experimental Brain Research 128 (4): 435–440. doi:10.1007/s002210050866. PMID 10541737.
- ↑ "Tussionex® Pennkinetic® (hydrocodone polistirex and chlorpheniramine polistirex) Extended-Release Suspension" (PDF). UCB. 2011.
- ↑ Gray, Shelly L.; Anderson, Melissa L.; Dublin, Sascha; Hanlon, Joseph T.; Hubbard, Rebecca; Walker, Rod; Yu, Onchee; Crane, Paul K.; Larson, Eric B. (January 26, 2015). "Cumulative Use of Strong Anticholinergics and Incident Dementia: A Prospective Cohort Study". JAMA Intern. Med. 175 (3): 401–7. doi:10.1001/jamainternmed.2014.7663. PMC 4358759. PMID 25621434. Retrieved January 27, 2015.
- ↑ Djerassi, Carl (1948). "Brominations with Pyridine Hydrobromide Perbromide". Journal of the American Chemical Society 70 (1): 417–418. doi:10.1021/ja01181a508.
| ||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||