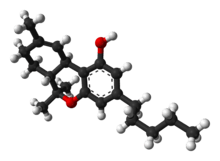
Tetrahydrocannabinol
 | |
 | |
| Systematic (IUPAC) name | |
|---|---|
|
(−)-(6aR,10aR)-6,6,9-Trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol | |
| Clinical data | |
| Trade names | Marinol |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status |
|
| Dependence liability | 8–10% (Relatively low risk of tolerance)[2] |
| Routes of administration | Orally, local/topical, transdermal, sublingual, inhaled |
| Pharmacokinetic data | |
| Bioavailability | 10–35% (inhalation), 6–20% (oral)[3] |
| Protein binding | 97–99%[3][4][5] |
| Metabolism | Mostly hepatic by CYP2C[3] |
| Biological half-life | 1.6–59 h,[3] 25–36 h (orally administered dronabinol) |
| Excretion | 65–80% (feces), 20–35% (urine) as acid metabolites[3] |
| Identifiers | |
| CAS Number |
1972-08-3 |
| ATC code | A04AD10 |
| PubChem | CID 16078 |
| IUPHAR/BPS | 2424 |
| DrugBank |
DB00470 |
| ChemSpider |
15266 |
| UNII |
7J8897W37S |
| ChEBI |
CHEBI:66964 |
| ChEMBL |
CHEMBL465 |
| Synonyms | Dronabinol |
| Chemical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g/mol |
| Specific rotation | -152° (ethanol) |
| |
| |
| Physical data | |
| Boiling point | 157 °C (315 °F) [6] |
| Solubility in water | 0.0028,[7] (23 °C) mg/mL (20 °C) |
| | |
Tetrahydrocannabinol (THC), or more precisely its main isomer (−)-trans-Δ9-tetrahydrocannabinol ( (6aR,10aR)-delta-9-tetrahydrocannabinol), is the principal psychoactive constituent (or cannabinoid) of cannabis. First isolated in 1964 by Israeli scientists Prof. Raphael Mechoulam and Dr. Yechiel Gaoni at the Weizmann Institute of Science[8][9][10] it is a water-clear glassy solid when cold, which becomes viscous and sticky if warmed. A pharmaceutical formulation of (−)-trans-Δ9-tetrahydrocannabinol, known by its INN dronabinol, is available by prescription in the U.S. and Canada under the brand name Marinol. An aromatic terpenoid, THC has a very low solubility in water, but good solubility in most organic solvents, specifically lipids and alcohols.[7] THC, CBD, CBN, CBC, CBG and about 80 other molecules make up the phytocannabinoid family.
Like most pharmacologically-active secondary metabolites of plants, THC in Cannabis is assumed to be involved in self-defense, perhaps against herbivores.[11] THC also possesses high UV-B (280–315 nm) absorption properties, which, it has been speculated, could protect the plant from harmful UV radiation exposure.[12][13][14]
Tetrahydrocannabinol, along with its double bond isomers and their stereoisomers, is one of only three cannabinoids scheduled by the Convention on Psychotropic Substances (the other two are dimethylheptylpyran and parahexyl). Previously listed as a substance under Schedule I of the 1971 Convention on Psychotropic Substances, THC was reclassified to Schedule II in 1991 following a recommendation from the World Health Organization. Based on their subsequent studies, the WHO has recommended the reclassification of THC to the less-stringent Schedule III.[15] Cannabis as a plant is scheduled by the Single Convention on Narcotic Drugs (Schedule I and IV).
Effects
THC has mild to moderate analgesic effects, and cannabis can be used to treat pain by altering transmitter release on dorsal root ganglion of the spinal cord and in the periaqueductal gray.[16] Other effects include relaxation, alteration of visual, auditory, and olfactory senses, fatigue, and appetite stimulation. THC has marked antiemetic properties. It may acutely reduce aggression.[17]
Due to its partial agonistic activity, THC appears to result in greater downregulation of cannabinoid receptors than endocannabinoids, further limiting its efficacy over other cannabinoids. While tolerance may limit the maximal effects of certain drugs, evidence suggests that tolerance develops irregularly for different effects with greater resistance for primary over side-effects, and may actually serve to enhance the drug's therapeutic window.[18] However, this form of tolerance appears to be irregular throughout mouse brain areas. THC, as well as other cannabinoids that contain a phenol group, possesses mild antioxidant activity sufficient to protect neurons against oxidative stress, such as that produced by glutamate-induced excitotoxicity.[19]
Appetite and taste
A study in mice suggested that based on the connection between palatable food and stimulation of dopamine (DA) transmission in the shell of the nucleus accumbens (NAc), cannabis may not only stimulate taste, but possibly the hedonic (pleasure) value of food as well. The study later demonstrates habitual use of THC lessening this heightened pleasure response, indicating a possible similarity in humans.[20] The inconsistency between DA habituation and enduring appetite observed after THC application suggests that cannabis-induced appetite stimulation is not only mediated by enhanced pleasure from palatable food, but through THC stimulation of another appetitive response as well.
Medical uses
In April 2014 the American Academy of Neurology published a systematic review of the efficacy and safety of medical marijuana and marijuana-derived products in certain neurological disorders.[21] The review identified 34 studies meeting inclusion criteria, of which 8 were rated as Class I quality.[21] The study found evidence supporting the effectiveness of cannabis extracts and THC in treating certain symptoms of multiple sclerosis, but found insufficient evidence to determine the effectiveness of cannabis products in treating several other neurological diseases.[21]
Multiple sclerosis symptoms
- Spasticity. Based on the results of 3 high quality trials and 5 of lower quality, oral cannabis extract was rated as effective, and THC as probably effective, for improving patient's subjective experience of spasticity. Oral cannabis extract and THC both were rated as possibly effective for improving objective measures of spasticity.[21]
- Centrally mediated pain and painful spasms. Based on the results of 4 high quality trials and 4 low quality trials, oral cannabis extract was rated as effective, and THC as probably effective in treating central pain and painful spasms.[21]
- Bladder dysfunction. Based on a single high quality study, oral cannabis extract and THC were rated as probably ineffective for controlling bladder complaints in multiple sclerosis[21]
Neurodegenerative disorders
- Huntington disease. No reliable conclusions could be drawn regarding the effectiveness of THC or oral cannabis extract in treating the symptoms of Huntington disease as the available trials were too small to reliably detect any difference[21]
- Parkinson disease. Based on a single study, oral cannabis extract was rated probably ineffective in treating levodopa-induced dyskinesia in Parkinson disease.[21]
- Alzheimer's disease. A 2011 Cochrane Review found insufficient evidence to conclude whether cannabis products have any utility in the treatment of Alzheimer's disease.[22]
Other neurological disorders
- Tourette syndrome. The available data was determined to be insufficient to allow reliable conclusions to be drawn regarding the effectiveness of oral cannabis extract or THC in controlling tics.[21]
- Cervical dystonia. Insufficient data was available to assess the effectiveness of oral cannabis extract of THC in treating cervical dystonia.[21]
- Epilepsy. Data was considered insufficient to judge the utility of cannabis products in reducing seizure frequency or severity.[21]
Other studies in humans
Evidence suggests that THC helps alleviate symptoms suffered both by AIDS patients, and by cancer patients undergoing chemotherapy, by increasing appetite and decreasing nausea.[23][24][25][26] It has also been shown to assist some glaucoma patients by reducing pressure within the eye, and is used in the form of cannabis by a number of multiple sclerosis patients, who use it to alleviate neuropathic pain and spasticity. The National Multiple Sclerosis Society is currently supporting further research into these uses.[27] THC has also shown antitumor activity in animal studies where it killed cancer cells.[28] Studies in humans have been limited by federal and state laws criminalizing marijuana.
In August 2009 a phase IV clinical trial by the Hadassah Medical Center in Jerusalem, Israel started to investigate the effects of THC on post-traumatic stress disorders.[29]
Studies have been conducted with spinal injury patients and THC.[30]
Both THC and CBD phytocannabinoids have been shown to decrease intestinal permeability.[31]
Adverse effects


Acute toxicity
There has never been a documented human fatality solely from overdosing on tetrahydrocannabinol.[32] However, numerous reports have suggested an association of cannabis smoking with an increased risk of myocardial infarction ,[33][34] also known as a heart attack but oral administration does not have this affect. Information about the toxicity of THC is primarily based on results from non-human studies. The toxicity depends on the route of administration and the laboratory animal.
The estimated lethal dose of intravenous dronabinol in humans is 30 mg/kg,[35] meaning lethality is unlikely. The typical medicinal dosage administered is two 2.5 mg capsules daily; for an 80 kg man (~170 lb). A lethal dose for such a person would be 960 of those capsules infused intravenously. Non-fatal overdoses have occurred: "Significant CNS symptoms in antiemetic studies followed oral doses of 0.4 mg/kg (28 mg/70 kg) of dronabinol capsules."[35]
- A meta analysis of cannabis and THC clinical trials conducted by the American Academy of Neurology found that of 1619 persons treated with cannabis products (including some treated with smoked cannabis and nabiximols), 6.9% discontinued due to side effects, compared to 2.2% of 1,118 treated with placebo. Detailed information regarding side effects was not available from all trials, but nausea, increased weakness, behavioral or mood changes, suicidal ideation, hallucinations, dizziness, and vasovagal symptoms, fatigue, and feelings of intoxication were each described as side effects in at least 2 trials. There was a single death rated by the investigator as "possibly related" to treatment. This person had a seizure followed by aspiration pneumonia. The paper does not describe whether this was one of the patients from the epilepsy trials.[21]
Cognitive effects
Its status as an illegal drug in most countries can make research difficult; for instance in the United States where the National Institute on Drug Abuse was the only legal source of cannabis for researchers until it recently became legalized in Colorado, Washington state, Oregon, Alaska, and Washington D.C.[36]
A 2011 systematic review evaluated published studies of the acute and long-term cognitive effects of cannabis. THC intoxication is well established to impair cognitive functioning on an acute basis, including effects on the ability to plan, organize, solve problems, make decisions, and control impulses. The extent of this impact may be greater in novice users, and paradoxically, those habituated to high level ingestion may have reduced cognition during withdrawal. Studies of long-term effects on cognition have provided conflicting results, with some studies finding no difference between long-term abstainers and never-users and others finding long-term deficits. The discrepancies between studies may reflect greater long-term effects among heavier users relative to occasional users, and greater duration of effect among those with heavy use as adolescents compared to later in life.[37] A second systematic review focused on neuroimaging studies found little evidence supporting an effect of cannabis use on brain structure and function.[38] A 2003 meta analysis concluded that any long-term cognitive effects were relatively modest in magnitude and limited to certain aspects of learning and memory.[39]
Impact on psychosis
A 2007 meta analysis concluded that cannabis use reduced the average age of onset of psychosis by 2.7 years relative to non-cannabis use.[40] A 2005 meta analysis concluded that adolescent use of cannabis increases the risk of psychosis, and that the risk is dose-related.[41] A 2004 literature review on the subject concluded that cannabis use is associated with a two-fold increase in the risk of psychosis, but that cannabis use is "neither necessary nor sufficient" to cause psychosis.[42] A French review from 2009 came to a conclusion that cannabis use, particularly that before age 15, was a factor in the development of schizophrenic disorders.[43]
Some studies have suggested that cannabis users have a greater risk of developing psychosis than non-users. This risk is most pronounced in cases with an existing risk of psychotic disorder.[44][45] A 2005 paper from the Dunedin study suggested an increased risk in the development of psychosis linked to polymorphisms in the COMT gene.[46] However, a more recent study cast doubt on the proposed connection between this gene and the effects of cannabis on the development of psychosis.[47]
A 2008 German review reported that cannabis was a causal factor in some cases of schizophrenia and stressed the need for better education among the public due to increasingly relaxed access to cannabis.[48]
Other potential long-term effects
A 2008 National Institutes of Health study of 19 chronic heavy marijuana users with cardiac and cerebral abnormalities (averaging 28 g to 272 g (1 to 9+ oz) weekly) and 24 controls found elevated levels of apolipoprotein C-III (apoC-III) in the chronic smokers.[49] An increase in apoC-III levels induces the development of hypertriglyceridemia.
Interactions
The effects of the drug can be reduced by the CB1 receptor inverse agonist rimonabant (SR141716A) as well as opioid receptor antagonists (opioid blockers) naloxone and naloxonazine.[20][50] The α7 nicotinic receptor antagonist methyllycaconitine can block self-administration of THC in rates comparable to the effects of varenicline on nicotine administration.[51]
Cannabidiol, the second most abundant cannabinoid found in cannabis, is an indirect antagonist against cannabinoid agonists; thus reducing the effects of anandamide and THC agonism on the CB1 and CB2 receptors.
Pharmacology
Mechanism of action
The actions of THC result from its partial agonist activity at the cannabinoid receptor CB1 (Ki=10nM[52]), located mainly in the central nervous system, and the CB2 receptor (Ki=24nM[52]), mainly expressed in cells of the immune system.[19] The psychoactive effects of THC are primarily mediated by its activation of CB1G-protein coupled receptors, which result in a decrease in the concentration of the second messenger molecule cAMP through inhibition of adenylate cyclase.[16]
The presence of these specialized cannabinoid receptors in the brain led researchers to the discovery of endocannabinoids, such as anandamide and 2-arachidonoyl glyceride (2-AG). THC targets receptors in a manner far less selective than endocannabinoid molecules released during retrograde signaling, as the drug has a relatively low cannabinoid receptor efficacy and affinity. In populations of low cannabinoid receptor density, THC may act to antagonize endogenous agonists that possess greater receptor efficacy.[18] THC is a lipophilic molecule[53] and may bind non-specifically to a variety of entities in the brain and body, such as adipose tissue (fat).[54][55]
THC, similarly to cannabidiol, albeit less potently, is an allosteric modulator of the μ- and δ-opioid receptors.[56]
Pharmacokinetics
THC is metabolized mainly to 11-OH-THC by the body. This metabolite is still psychoactive and is further oxidized to 11-nor-9-carboxy-THC (THC-COOH). In humans and animals, more than 100 metabolites could be identified, but 11-OH-THC and THC-COOH are the dominating metabolites. Metabolism occurs mainly in the liver by cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP3A4.[57] More than 55% of THC is excreted in the feces and ~20% in the urine. The main metabolite in urine is the ester of glucuronic acid and THC-COOH and free THC-COOH. In the feces, mainly 11-OH-THC was detected.[58]
Physical and chemical properties
Discovery and structure identification
The discovery of THC by a team of researchers from Hebrew University Pharmacy School was first described in "Isolation, structure and partial synthesis of an active constituent of hashish", published in the Journal of the American Chemical Society in 1964.[8] Research was also published in the academic journal Science, with "Marijuana chemistry" by Raphael Mechoulam in June 1970.[59]
Isomerism

| 7 double bond isomers and their 30 stereoisomers | ||||||||
|---|---|---|---|---|---|---|---|---|
| Dibenzopyran numbering | Monoterpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ6a,7-tetrahydrocannabinol | 9 and 10a | 8,9,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ4-tetrahydrocannabinol | 1 and 3 | 4 | No | Schedule I |  |
| Δ7-tetrahydrocannabinol | 6a, 9 and 10a | 6a,9,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ5-tetrahydrocannabinol | 1, 3 and 4 | 8 | No | Schedule I | 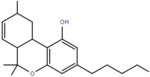 |
| Δ8-tetrahydrocannabinol | 6a and 10a | 6a,7,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ6-tetrahydrocannabinol | 3 and 4 | 4 | Yes | Schedule I | 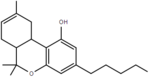 |
| Δ9,11-tetrahydrocannabinol | 6a and 10a | 6a,7,8,9,10,10a-hexahydro-6,6-dimethyl-9-methylene-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ1,7-tetrahydrocannabinol | 3 and 4 | 4 | No | Schedule I | 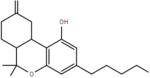 |
| Δ9-tetrahydrocannabinol | 6a and 10a | 6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ1-tetrahydrocannabinol | 3 and 4 | 4 | Yes | Schedule II | 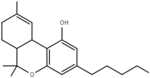 |
| Δ10-tetrahydrocannabinol | 6a and 9 | 6a,7,8,9-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ2-tetrahydrocannabinol | 1 and 4 | 4 | No | Schedule I |  |
| Δ6a,10a-tetrahydrocannabinol | 9 | 7,8,9,10-tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]pyran-1-ol | Δ3-tetrahydrocannabinol | 1 | 2 | No | Schedule I | 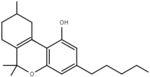 |
| 4 stereoisomers of Δ9-tetrahydrocannabinol | ||||
|---|---|---|---|---|
| Names | Description | Natural occurrence | Structure | |
| (−)-trans-Δ9-tetrahydrocannabinol | (6aR,10aR)-Δ9-tetrahydrocannabinol | levorotary trans | Yes | -(6aR%2C10aR)-%CE%949-Tetrahydrocannabinol_(with_hydrogen_atoms_shown).svg.png) |
| (−)-cis-Δ9-tetrahydrocannabinol | (6aS,10aR)-Δ9-tetrahydrocannabinol | levorotary cis | Yes | -(6aS%2C10aR)-%CE%949-Tetrahydrocannabinol_(with_hydrogen_atoms_shown).svg.png) |
| (+)-trans-Δ9-tetrahydrocannabinol | (6aS,10aS)-Δ9-tetrahydrocannabinol | dextrorotary trans | No | -(6aS%2C10aS)-%CE%949-Tetrahydrocannabinol_(with_hydrogen_atoms_shown).svg.png) |
| (+)-cis-Δ9-tetrahydrocannabinol | (6aR,10aS)-Δ9-tetrahydrocannabinol | dextrorotary cis | No | -(6aR%2C10aS)-%CE%949-Tetrahydrocannabinol_(with_hydrogen_atoms_shown).svg.png) |
Note that 6H-dibenzo[b,d]pyran-1-ol is the same as 6H-benzo[c]chromen-1-ol.
- Further reading on cannabanoid isomerism: John C. Leffingwell (May 2003). "Chirality & Bioactivity I.: Pharmacology" (PDF). pp. 18–20. Retrieved 12 January 2014.
Biosynthesis
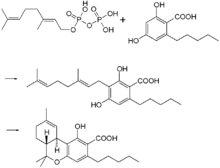
In the Cannabis plant, THC occurs mainly as tetrahydrocannabinolic acid (THCA, 2-COOH-THC). Geranyl pyrophosphate and olivetolic acid react, catalysed by an enzyme to produce cannabigerolic acid,[60] which is cyclized by the enzyme THC acid synthase to give THCA. Over time, or when heated, THCA is decarboxylated, producing THC. The pathway for THCA biosynthesis is similar to that which produces the bitter acid humulone in hops.[61][62]
Detection in body fluids
THC, 11-OH-THC and THC-COOH can be detected and quantified in blood, urine, hair, oral fluid or sweat using a combination of immunoassay and chromatographic techniques as part of a drug use testing program or in a forensic investigation of a traffic or other criminal offense or suspicious death.[63][64][65]
History
Since at least 1986, the trend has been for THC in general, and especially the Marinol preparation, to be downgraded to less and less stringently-controlled schedules of controlled substances, in the U.S. and throughout the rest of the world.
On May 13, 1986, the Drug Enforcement Administration (DEA) issued a Final Rule and Statement of Policy authorizing the "Rescheduling of Synthetic Dronabinol in Sesame Oil and Encapsulated in Soft Gelatin Capsules From Schedule I to Schedule II" (DEA 51 FR 17476-78). This permitted medical use of Marinol, albeit with the severe restrictions associated with Schedule II status.[66] For instance, refills of Marinol prescriptions were not permitted. At its 1045th meeting, on April 29, 1991, the Commission on Narcotic Drugs, in accordance with article 2, paragraphs 5 and 6, of the Convention on Psychotropic Substances, decided that Δ9-tetrahydrocannabinol (also referred to as Δ9-THC) and its stereochemical variants should be transferred from Schedule I to Schedule II of that Convention. This released Marinol from the restrictions imposed by Article 7 of the Convention (See also United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances).
An article published in the April–June 1998 issue of the Journal of Psychoactive Drugs found that "Healthcare professionals have detected no indication of scrip-chasing or doctor-shopping among the patients for whom they have prescribed dronabinol". The authors state that Marinol has a low potential for abuse.[67]
In 1999, Marinol was rescheduled from Schedule II to III of the Controlled Substances Act, reflecting a finding that THC had a potential for abuse less than that of cocaine and heroin. This rescheduling constituted part of the argument for a 2002 petition for removal of cannabis from Schedule I of the Controlled Substances Act, in which petitioner Jon Gettman noted, "Cannabis is a natural source of dronabinol (THC), the ingredient of Marinol, a Schedule III drug. There are no grounds to schedule cannabis in a more restrictive schedule than Marinol".[68]
At its 33rd meeting, in 2003, the World Health Organization Expert Committee on Drug Dependence recommended transferring THC to Schedule IV of the Convention, citing its medical uses and low abuse potential.[69]
Society and culture
Brand names
Dronabinol is the INN for a pure isomer of THC, (–)-trans-Δ9-tetrahydrocannabinol,[70] which is the main isomer found in cannabis. Synthesized dronabinol is marketed as Marinol[71] (a registered trademark of Solvay Pharmaceuticals). Dronabinol is also marketed, sold, and distributed by PAR Pharmaceutical Companies under the terms of a license and distribution agreement with SVC pharma LP, an affiliate of Rhodes Technologies. Synthesized THC may be generally referred to as dronabinol. It is available as a prescription drug (under Marinol[72]) in several countries including the United States, Germany, South Africa and Australia.[73] In the United States, Marinol is a Schedule III drug, available by prescription, considered to be non-narcotic and to have a low risk of physical or mental dependence. Efforts to get cannabis rescheduled as analogous to Marinol have not succeeded thus far, though a 2002 petition has been accepted by the DEA. As a result of the rescheduling of Marinol from Schedule II to Schedule III, refills are now permitted for this substance. Marinol has been approved by the U.S. Food and Drug Administration (FDA) in the treatment of anorexia in HIV/AIDS patients, as well as for refractory nausea and vomiting of patients undergoing chemotherapy, which has raised much controversy[74] as to why natural THC is considered a schedule I drug.[75]
An overdose usually presents with lethargy, decreased motor coordination, slurred speech, and postural hypotension. The FDA estimates the lethal human dose of intravenous dronabinol to be 30 mg/kg (2100 mg/ 70 kg).[76]
An analog of dronabinol, nabilone, is available commercially in Canada under the trade name Cesamet, manufactured by Valeant Pharmaceuticals. Cesamet has also received FDA approval and began marketing in the U.S. in 2006. Nabilone is a Schedule II drug.[77]
Comparisons with medical cannabis
Female cannabis plants contain more than 60 cannabinoids, including cannabidiol (CBD), thought to be the major anticonvulsant that helps multiple sclerosis patients;[78] and cannabichromene (CBC), an anti-inflammatory which may contribute to the pain-killing effect of cannabis.[79]
It takes over one hour for Marinol to reach full systemic effect,[80] compared to seconds or minutes for smoked or vaporized cannabis.[81] Some patients accustomed to inhaling just enough cannabis smoke to manage symptoms have complained of too-intense intoxication from Marinol's predetermined dosages. Many patients have said that Marinol produces a more acute psychedelic effect than cannabis, and it has been speculated that this disparity can be explained by the moderating effect of the many non-THC cannabinoids present in cannabis. For that reason, alternative THC-containing medications based on botanical extracts of the cannabis plant such as nabiximols are being developed. Mark Kleiman, director of the Drug Policy Analysis Program at UCLA's School of Public Affairs said of Marinol, "It wasn't any fun and made the user feel bad, so it could be approved without any fear that it would penetrate the recreational market, and then used as a club with which to beat back the advocates of whole cannabis as a medicine."[82] Mr. Kleiman's opinion notwithstanding, clinical trials comparing the use of cannabis extracts with Marinol in the treatment of cancer cachexia have demonstrated equal efficacy and well-being among patients in the two treatment arms.[83] United States federal law currently registers dronabinol as a Schedule III controlled substance, but all other cannabinoids remain Schedule I, except synthetics like nabilone.[84]
See also
- Cannabinoids
- 11-Hydroxy-THC, metabolite of THC
- Anandamide, 2-Arachidonoylglycerol, endogenous cannabinoid agonists
- Cannabidiol (CBD), an isomer of THC
- Cannabinol (CBN), a metabolite of THC
- Dimethylheptylpyran
- Parahexyl
- Tetrahydrocannabinolic acid, the biosynthetic precursor for THC
- HU-210, WIN 55,212-2, JWH-133, synthetic cannabinoid agonists
- Medical cannabis
- War on Drugs
- Effects of cannabis
References
- ↑ http://www.fda.gov/ohrms/dockets/dockets/05n0479/05N-0479-emc0004-04.pdf
- ↑ Marlowe, Douglas B. (December 2010). "The Facts On Marijuana". NADCP.
Based upon several nationwide epidemiological studies, marijuana’s dependence liability has been reliably determined to be 8 to 10 percent.
- 1 2 3 4 5 Grotenhermen, F (2003). "Pharmacokinetics and pharmacodynamics of cannabinoids". Clin Pharmacokinet 42 (4): 327–60. doi:10.2165/00003088-200342040-00003. PMID 12648025. (subscription required (help)).
- ↑ The Royal Pharmaceutical Society of Great Britain (30 November 2006). "Cannabis". In Sean C. Sweetman. Martindale: The Complete Drug Reference: Single User (35th ed.). Pharmaceutical Press. ISBN 978-0-85369-703-9.
- ↑ "Tetrahydrocannabinol – Compound Summary". National Center for Biotechnology Information. PubChem. Retrieved 12 January 2014.
Dronabinol has a large apparent volume of distribution, approximately 10 L/kg, because of its lipid solubility. The plasma protein binding of dronabinol and its metabolites is approximately 97%.
- ↑ McPartland JM, Russo EB (2001). "Cannabis and cannabis extracts: greater than the sum of their parts?" (PDF). Journal of Cannabis Therapeutics 1 (3/4): 103–132. doi:10.1300/J175v01n03_08.
- 1 2 Garrett ER, Hunt CA (July 1974). "Physicochemical properties, solubility, and protein binding of Δ9-tetrahydrocannabinol". J. Pharm. Sci. 63 (7): 1056–64. doi:10.1002/jps.2600630705. PMID 4853640.
- 1 2 Gaoni Y, Mechoulam R (1964). "Isolation, structure and partial synthesis of an active constituent of hashish". Journal of the American Chemical Society 86 (8): 1646–1647. doi:10.1021/ja01062a046.
- ↑ "Interview with the winner of the first ECNP Lifetime Achievement Award: Raphael Mechoulam, Israel". February 2007.
- ↑ Geller, Tom (2007). "Cannabinoids: A Secret History". Chemical Heritage Newsmagazine 25 (2). Archived from the original on 19 June 2008.
- ↑ Pate, David W. (1994). "Chemical ecology of Cannabis". Journal of the International Hemp Association 1 (29): 32–37.
- ↑ Pate, David W. (1983). "Possible role of ultraviolet radiation in evolution of Cannabis chemotypes". Economic Botany 37 (4): 396–405. doi:10.1007/BF02904200.
- ↑ Lydon, John; Teramura, Alan H. (1987). "Photochemical decomposition of cannabidiol in its resin base". Phytochemistry 26 (4): 1216–1217. doi:10.1016/S0031-9422(00)82388-2.
- ↑ Lydon J, Teramura AH, Coffman CB (1987). "UV-B radiation effects on photosynthesis, growth and cannabinoid production of two Cannabis sativa chemotypes". Photochemistry and Photobiology 46 (2): 201–206. doi:10.1111/j.1751-1097.1987.tb04757.x. PMID 3628508.
- ↑ A Primer on the UN Drug Control Conventions. Transnational Institute
- 1 2 Elphick MR, Egertová M (2001). "The neurobiology and evolution of cannabinoid signalling". Philosophical Transactions of the Royal Society B: Biological Sciences 356 (1407): 381–408. doi:10.1098/rstb.2000.0787. PMC 1088434. PMID 11316486.
- ↑ Hoaken PN, Stewart SH (2003). "Drugs of abuse and the elicitation of human aggressive behavior". Addictive Behaviors 28 (9): 1533–1554. doi:10.1016/j.addbeh.2003.08.033. PMID 14656544.
- 1 2 Pertwee RG (2008). "The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin". British Journal of Pharmacology 153 (2): 199–215. doi:10.1038/sj.bjp.0707442. PMC 2219532. PMID 17828291.
- 1 2 Pertwee RG (2006). "The pharmacology of cannabinoid receptors and their ligands: An overview". International Journal of Obesity 30: S13–S18. doi:10.1038/sj.ijo.0803272. PMID 16570099.
- 1 2 De Luca MA, Solinas M, Bimpisidis Z, Goldberg SR, Di Chiara G (2011). "Cannabinoid facilitation of behavioral and biochemical hedonic taste responses". Neuropharmacology 63 (1): 161–168. doi:10.1016/j.neuropharm.2011.10.018. PMC 3705914. PMID 22063718.
- 1 2 3 4 5 6 7 8 9 10 11 12 Koppel BS, Brust JC, Fife T, Bronstein J, Youssof S, Gronseth G, Gloss D (April 2014). "Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders: Report of the Guideline Development Subcommittee of the American Academy of Neurology". Neurology 82 (17): 1556–63. doi:10.1212/WNL.0000000000000363. PMC 4011465. PMID 24778283.
- ↑ Krishnan S, Cairns R, Howard R (2009). Krishnan, Sarada, ed. "Cochrane Database of Systematic Reviews". Cochrane database of systematic reviews (Online) (2): CD007204. doi:10.1002/14651858.CD007204.pub2. PMID 19370677.
|chapter=ignored (help) - ↑ "Cannabis and Cannabinoids". National Cancer Institute. Retrieved 12 January 2014.
- ↑ Haney M, Gunderson EW, Rabkin J, Hart CL, Vosburg SK, Comer SD, Foltin RW (2007). "Dronabinol and marijuana in HIV-positive marijuana smokers. Caloric intake, mood, and sleep". Journal of Acquired Immune Deficiency Syndromes 45 (5): 545–54. doi:10.1097/QAI.0b013e31811ed205. PMID 17589370.
- ↑ Abrams DI, Hilton JF, Leiser RJ, Shade SB, Elbeik TA, Aweeka FT, Benowitz NL, Bredt BM, Kosel B, Aberg JA, Deeks SG, Mitchell TF, Mulligan K, Bacchetti P, McCune JM, Schambelan M (2003). "Short-term effects of cannabinoids in patients with HIV-1 infection: a randomized, placebo-controlled clinical trial". Annals of Internal Medicine 139 (4): 258–66. doi:10.7326/0003-4819-139-4-200308190-00008. PMID 12965981.
- ↑ Grotenhermen, Franjo; Russo, Ethan, eds. (2002). "Review of Therapeutic Effects". Cannabis and Cannabinoids: Pharmacology, Toxicology and Therapeutic Potential. New York City: Psychology Press. p. 124. ISBN 978-0-7890-1508-2.
The only approved preparations to date, Marinol (dronabinol, Δ9-THC) and Cesamet (nabilone), are approved for the indication of nausea and vomiting associated with cancer chemotherapy. Marinol is also approved for anorexia and cachexia in HIV/AIDS.
- ↑ "Marijuana (Cannabis)". National Multiple Sclerosis Society. Retrieved 5 September 2009.
- ↑ http://www.cancer.gov/about-cancer/treatment/cam/patient/cannabis-pdq/#link/_13
- ↑ Clinical trial number NCT00965809 for "Add on Study on Δ9-THC Treatment for Posttraumatic Stress Disorders (PTSD) (THC_PTSD)" at ClinicalTrials.gov
- ↑ Hannigan WC, Destree R, Truong XT (1986). "The effect of delta-9-THC on human spasticity" (PDF). American Society for Clinical Pharmacology and Therapeutics, Eighty-seventh Annual Meeting, March 20–22, 1986 39 (2): 198, abstract B45.
- ↑ SCLabs, Leaky Gut Syndrome: Cannabinoids and the Endocannabinoid System (ECS) as a therapeutic target
- ↑ Walker JM, Huang SM (August 2002). "Cannabinoid analgesia". Pharmacol. Ther. 95 (2): 127–35. doi:10.1016/S0163-7258(02)00252-8. PMID 12182960.
...to date, there are no deaths known to have resulted from overdose of cannabis. (p. 128)
- ↑ Thomas G, Kloner RA, Rezkalla S (January 2014). "Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know". Am. J. Cardiol. 113 (1): 187–90. doi:10.1016/j.amjcard.2013.09.042. PMID 24176069.
- ↑ Aryana A, Williams MA (May 2007). "Marijuana as a trigger of cardiovascular events: speculation or scientific certainty?". Int. J. Cardiol. 118 (2): 141–4. doi:10.1016/j.ijcard.2006.08.001. PMID 17005273.
- 1 2 "DRONABINOL capsule [American Health Packaging]". National Library of Medicine. Daily Med. July 2012. Retrieved 12 January 2014.
The estimated lethal human dose of intravenous dronabinol is 30 mg/kg (2100 mg/70 kg). Significant CNS symptoms in antiemetic studies followed oral doses of 0.4 mg/kg (28 mg/70 kg) of dronabinol capsules.
- ↑ "Medical Marijuana". Multidisciplinary Association for Psychoactive Substances. Retrieved 12 January 2014.
- ↑ Crean RD, Crane NA, Mason BJ (March 2011). "An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions". J Addict Med 5 (1): 1–8. doi:10.1097/ADM.0b013e31820c23fa. PMC 3037578. PMID 21321675.
- ↑ Martín-Santos R, Fagundo AB, Crippa JA, Atakan Z, Bhattacharyya S, Allen P, Fusar-Poli P, Borgwardt S, Seal M, Busatto GF, McGuire P (March 2010). "Neuroimaging in cannabis use: a systematic review of the literature". Psychol Med 40 (3): 383–98. doi:10.1017/S0033291709990729. PMID 19627647.
- ↑ Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T (2003). "Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study". Journal of the International Neuropsychological Society 9 (5): 679–89. doi:10.1017/S1355617703950016. PMID 12901774. Lay summary – WebMD (1 July 2003).
- ↑ Large M, Sharma S, Compton MT, Slade T, Nielssen O (June 2011). "Cannabis use and earlier onset of psychosis: a systematic meta-analysis". Arch. Gen. Psychiatry 68 (6): 555–61. doi:10.1001/archgenpsychiatry.2011.5. PMID 21300939.
- ↑ Semple DM, McIntosh AM, Lawrie SM (March 2005). "Cannabis as a risk factor for psychosis: systematic review". J. Psychopharmacol. (Oxford) 19 (2): 187–94. doi:10.1177/0269881105049040. PMID 15871146.
- ↑ Arseneault L, Cannon M, Witton J, Murray RM (2004). "Causal association between cannabis and psychosis: examination of the evidence". The British Journal of Psychiatry 184 (2): 110–117. doi:10.1192/bjp.184.2.110. PMID 14754822.
- ↑ Laqueille X (2009). "Le cannabis est-il un facteur de vulnérabilité des troubles schizophrènes?" [Is cannabis is a vulnerability factor of schizophrenic disorders?]. Archives de Pédiatrie 16 (9): 1302–5. doi:10.1016/j.arcped.2009.03.016. PMID 19640690. (registration required (help)).
- ↑ Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G (2007). "Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review". The Lancet 370 (9584): 319–28. doi:10.1016/S0140-6736(07)61162-3. PMID 17662880.
- ↑ Henquet C, Krabbendam L, Spauwen J, Kaplan C, Lieb R, Wittchen HU, van Os J (2005). "Prospective cohort study of cannabis use, predisposition for psychosis, and psychotic symptoms in young people". BMJ 330 (7481): 11–0. doi:10.1136/bmj.38267.664086.63. PMC 539839. PMID 15574485.
- ↑ Caspi A, Moffitt TE, Cannon M, McClay J, Murray R, Harrington H, Taylor A, Arseneault L, Williams B, Braithwaite A, Poulton R, Craig IW (2005). "Moderation of the Effect of Adolescent-Onset Cannabis Use on Adult Psychosis by a Functional Polymorphism in the Catechol-O-Methyltransferase Gene: Longitudinal Evidence of a Gene X Environment Interaction". Biological Psychiatry 57 (10): 1117–27. doi:10.1016/j.biopsych.2005.01.026. PMID 15866551.
- ↑ Zammit S, Spurlock G, Williams H, Norton N, Williams N, O'Donovan MC, Owen MJ; Spurlock (2007). "Genotype effects of CHRNA7, CNR1 and COMT in schizophrenia: interactions with tobacco and cannabis use". The British Journal of Psychiatry 191 (5): 402–7. doi:10.1192/bjp.bp.107.036129. PMID 17978319. Lay summary – MedWireNews.
- ↑ Kawohl W, Rössler W (2008). "Cannabis and Schizophrenia: new findings in an old debate". Neuropsychiatrie : Klinik, Diagnostik, Therapie und Rehabilitation : Organ der Gesellschaft Osterreichischer Nervenarzte und Psychiater 22 (4): 223–9. PMID 19080993.
- ↑ Jayanthi S, Buie S, Moore S, Herning RI, Better W, Wilson NM, Contoreggi C, Cadet JL (2008). "Heavy marijuana users show increased serum apolipoprotein C-III levels: evidence from proteomic analyses". Molecular Psychiatry 15 (1): 101–112. doi:10.1038/mp.2008.50. PMC 2797551. PMID 18475272. Lay summary – Reuters (May 13, 2008).
- ↑ Lupica CR, Riegel AC, Hoffman AF (2004). "Marijuana and cannabinoid regulation of brain reward circuits". British Journal of Pharmacology 143 (2): 227–34. doi:10.1038/sj.bjp.0705931. PMC 1575338. PMID 15313883.
- ↑ Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, Fratta W, Goldberg SR (2007). "Nicotinic 7 Receptors as a New Target for Treatment of Cannabis Abuse". Journal of Neuroscience 27 (21): 5615–20. doi:10.1523/JNEUROSCI.0027-07.2007. PMID 17522306. Lay summary – New Scientist (22 May 2007).
- 1 2 "PDSP Database – UNC". NIMH Psychoactive Drug Screening Program. Retrieved 11 June 2013.
- ↑ Rashidi H, Akhtar MT, van der Kooy F, Verpoorte R, Duetz WA (November 2009). "Hydroxylation and Further Oxidation of Δ9-Tetrahydrocannabinol by Alkane-Degrading Bacteria" (PDF). Appl Environ Microbiol 75 (22): 7135–7141. doi:10.1128/AEM.01277-09. PMC 2786519. PMID 19767471.
Δ9-THC and many of its derivatives are highly lipophilic and poorly water soluble. Calculations of the n-octanol/water partition coefficient (Ko/w) of Δ9-THC at neutral pH vary between 6,000, using the shake flask method, and 9.44 × 106, by reverse-phase high-performance liquid chromatography estimation.
- ↑ Ashton CH (February 2001). "Pharmacology and effects of cannabis: a brief review". Br J Psychiatry 178 (2): 101–106. doi:10.1192/bjp.178.2.101. PMID 11157422.
Because they are extremely lipid soluble, cannabinoids accumulate in fatty tissues, reaching peak concentrations in 4–5 days. They are then slowly released back into other body compartments, including the brain. ... Within the brain, THC and other cannabinoids are differentially distributed. High concentrations are reached in neocortical, limbic, sensory and motor areas.
- ↑ Huestis MA (August 2007). "Human cannabinoid pharmacokinetics". Chem Biodivers 4 (8): 1770–804. doi:10.1002/cbdv.200790152. PMC 2689518. PMID 17712819.
THC is highly lipophilic and initially taken up by tissues that are highly perfused, such as the lung, heart, brain, and liver.
- ↑ Kathmann M, Flau K, Redmer A, Tränkle C, Schlicker E (February 2006). "Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors". Naunyn Schmiedebergs Arch. Pharmacol. 372 (5): 354–61. doi:10.1007/s00210-006-0033-x. PMID 16489449.
- ↑ Watanabe K, Yamaori S, Funahashi T, Kimura T, Yamamoto I (March 2007). "Cytochrome P450 enzymes involved in the metabolism of tetrahydrocannabinols and cannabinol by human hepatic microsomes". Life Science 80 (15): 1415–9. doi:10.1016/j.lfs.2006.12.032. PMID 17303175.
- ↑ Huestis MA (2005). "Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannabinol, Cannabidiol and Cannabinol". Cannabinoids. Handbook of Experimental Pharmacology 168 (168): 657–90. doi:10.1007/3-540-26573-2_23. ISBN 3-540-22565-X. PMID 16596792.
- ↑ Mechoulam R (1970). "Marihuana chemistry". Science 168 (3936): 1159–66. Bibcode:1970Sci...168.1159M. doi:10.1126/science.168.3936.1159. PMID 4910003.
- ↑ Fellermeier M, Zenk MH (1998). "Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol". FEBS Letters 427 (2): 283–5. doi:10.1016/S0014-5793(98)00450-5. PMID 9607329.
- ↑ Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, Gang DR, Weiblen GD, Dixon RA (2009). "Identification of candidate genes affecting Δ9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany 60 (13): 3715–26. doi:10.1093/jxb/erp210. PMC 2736886. PMID 19581347.
- ↑ Baker PB, Taylor BJ, Gough TA (June 1981). "The tetrahydrocannabinol and tetrahydrocannabinolic acid content of cannabis products". J Pharm Pharmacol. 33 (6): 369–72. doi:10.1111/j.2042-7158.1981.tb13806.x. PMID 6115009.
- ↑ Schwilke EW, Schwope DM, Karschner EL, Lowe RH, Darwin WD, Kelly DL, Goodwin RS, Gorelick DA, Huestis MA (2009). "Δ9-Tetrahydrocannabinol (THC), 11-Hydroxy-THC, and 11-Nor-9-carboxy-THC Plasma Pharmacokinetics during and after Continuous High-Dose Oral THC". Clinical Chemistry 55 (12): 2180–2189. doi:10.1373/clinchem.2008.122119. PMC 3196989. PMID 19833841.
- ↑ Röhrich J, Schimmel I, Zörntlein S, Becker J, Drobnik S, Kaufmann T, Kuntz V, Urban R (2010). "Concentrations of Δ9-Tetrahydrocannabinol and 11-Nor-9-Carboxytetrahydrocannabinol in Blood and Urine After Passive Exposure to Cannabis Smoke in a Coffee Shop". Journal of Analytical Toxicology 34 (4): 196–203. doi:10.1093/jat/34.4.196. PMID 20465865.
- ↑ Baselt, R. (2011). Disposition of Toxic Drugs and Chemicals in Man (9th ed.). Seal Beach, CA: Biomedical Publications. pp. 1644–8.
- ↑ 51 Fed. Reg. 17476 (1986), Tuesday, May 13, 1986, pages 17476-17478
- ↑ Calhoun SR, Galloway GP, Smith DE (1998). "Abuse potential of dronabinol (Marinol)". Journal of Psychoactive Drugs 30 (2): 187–96. doi:10.1080/02791072.1998.10399689. PMID 9692381.
- ↑ "Petition to Reschedule Cannabis (Marijuana)" (PDF). Coalition for Rescheduling Cannabis. 9 October 2002.
- ↑ "WHO Expert Committee on Drug Dependence". World Health Organization. Retrieved 12 January 2014.
- ↑ "List of psychotropic substances under international control" (PDF). International Narcotics Control Board. p. 5. Archived from the original (PDF) on 7 September 2005. Retrieved 20 April 2011.
This international non-proprietary name refers to only one of the stereochemical variants of delta-9-tetrahydrocannabinol, namely (−)-trans-delta-9-tetrahydrocannabinol
- ↑ EMCDDA, ELDD Comparative Study, May 2002.
- ↑ "Marinol – the Legal Medical Use for the Marijuana Plant". Drug Enforcement Administration. Archived from the original on 21 October 2002. Retrieved 20 April 2011.
- ↑ Alchimia Blog, Marijuana and Medicine: Cesamet, Marinol, Sativex
- ↑ Downs, David (21 October 2014). "War on marijuana unconstitutional, doctors testify in federal court Monday". sfgate.com. Retrieved 21 October 2014.
- ↑ Eustice, Carol (12 August 1997). "Medicinal Marijuana: A Continuing Controversy". About.com. Retrieved 20 April 2011.
- ↑ "Marinol" (PDF). FDA.gov. Retrieved 14 March 2014.
- ↑ "Title 21 of the Code of Federal Regulations – PART 1308 — SCHEDULES OF CONTROLLED SUBSTANCES". US Department of Justice. DEA Office of Diversion Control. Retrieved 12 January 2014. With changes through 77 FR 4235 (January 27, 2012).
- ↑ Pickens JT (1981). "Sedative activity of cannabis in relation to its delta'-trans-tetrahydrocannabinol and cannabidiol content". British Journal of Pharmacology 72 (4): 649–56. doi:10.1111/j.1476-5381.1981.tb09145.x. PMC 2071638. PMID 6269680.
- ↑ Burns TL, Ineck JR (2006). "Cannabinoid Analgesia as a Potential New Therapeutic Option in the Treatment of Chronic Pain". Annals of Pharmacotherapy 40 (2): 251–260. doi:10.1345/aph.1G217. PMID 16449552.
- ↑ MARINOL (dronabinol) capsule drug label/data at Daily Med from U.S. National Library of Medicine, National Institutes of Health.
- ↑ McKim, William A (2002). Drugs and Behavior: An Introduction to Behavioral Pharmacology (5th ed.). Prentice Hall. p. 400. ISBN 0-13-048118-1.
- ↑ Greenberg, Gary (1 November 2005). "Respectable Reefer". Mother Jones. Retrieved 8 April 2010.
- ↑ "Cannabis and Cannabinoids (PDQ®)". Cancer Topics. National Cancer Institute, U.S. Department of Health and Human Services.
- ↑ "Government eases restrictions on pot derivative". Online Athens. Retrieved 12 January 2014.
External links
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||
| ||||||||||
| ||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||
