Hydroxyprogesterone caproate
 | |
| Systematic (IUPAC) name | |
|---|---|
|
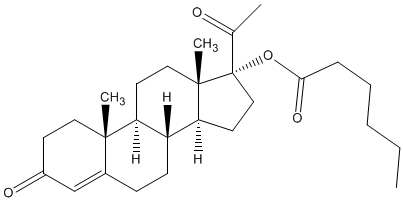
[(8R,9S,10R,13S,14S,17R)-17-acetyl-10,13-dimethyl- 3-oxo-2,6,7,8,9,11,12,14,15,16-decahydro- 1H-cyclopenta[a]phenanthren-17-yl] hexanoate | |
| Clinical data | |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection |
| Pharmacokinetic data | |
| Biological half-life | 7.8 days |
| Identifiers | |
| CAS Number |
630-56-8 |
| ATC code | G03DA03 |
| PubChem | CID 169870 |
| ChemSpider |
148552 |
| Chemical data | |
| Formula | C27H40O4 |
| Molar mass | 428.6041 g/mol |
| |
| |
| | |
Hydroxyprogesterone caproate (INN, USAN, JAN), or 17α-hydroxyprogesterone caproate, also known as hydroxyprogesterone hexanoate (BANM), marketed under brand names including Delalutin, Proluton, Makena, Prodrox, Hylutin, and many others, is a steroidal progestin (that is, a synthetic progestogen) and 17α-hydroxyprogesterone derivative that is related to other derivatives of 17α-hydroxyprogesterone such as medroxyprogesterone acetate and megestrol acetate.[1] It is an ester of 17α-hydroxyprogesterone formed from caproic acid (hexanoic acid).[1]
Hydroxyprogesterone caproate was previously marketed under the trade name Delalutin by Squibb, which was approved by the United States (U.S.) Food and Drug Administration (FDA) in 1956 and withdrawn from marketing in 1999. It is also sold as Proluton throughout Europe.[1]
The U.S. FDA approved Makena from KV Pharmaceutical (previously named as Gestiva) on February 4, 2011 for prevention of preterm delivery in women with a history of preterm delivery, sparking a pricing controversy.
Safety
The use of hydroxyprogesterone caproate in pregnancy to prevent preterm birth in women with a history of preterm delivery between 20 weeks and 36 weeks and 6 days is supported by the Society of Maternal Fetal Medicine Clinic Guidelines put out in May 2012 as Level I and III evidence, Level A recommendation.[2] Level I evidence refers to a properly powered randomized controlled trial, and level III evidence is support from expert opinion, while a Level A recommendation confers that the recommendation is made based on good and consistent scientific evidence. Hydroxyprogesterone caproate 250 mg IM weekly preferably starting at 16–20 weeks until 36 weeks is recommended. In these women, if the transvaginal ultrasound cervical length shortens to <25 mm at < 24 weeks, cervical cerclage may be offered. In the 2013 study the guideline recommendation is based on by [3] there was also a significant decrease of neonatal morbidity including lower rates of necrotizing enterocolitis (0 in treatment group vs 4 in control), intraventricular hemorrhage (4 in the treatment group compared with 8 in the control for relative risk of 0.25), and need for supplemental oxygen (14% in treatment group vs 24% in placebo for relative risk of 0.42). Furthermore, this study contained 463 patients, 310 of whom received injection. Of these patients, 9 had infants with congenital malformations (2%), but there was no consistent pattern and none involved internal organs.
Hydroxyprogesterone caproate is currently (as of June 2014) pregnancy category B, meaning there is no evidence of fetal risk with use of this drug during pregnancy. Although this is now the recommendation, this has not always been the case. A 2006 Cochrane Review concluded "...important maternal and infant outcomes have been poorly reported to date... information regarding the potential harms of progesterone therapy to prevent preterm birth is limited".[4] There was a similar conclusion from a review by Marc Keirse of Flinders University.[5] Three clinical studies in singleton pregnancies of 250 mg/week of intramuscular hydroxyprogesterone caproate have all shown a trend for an increase in pregnancy loss due to miscarriage compared to placebo.[6][7][8][9] The FDA expressed concern about miscarriage at the 2006 advisory committee meeting; the committee voted unanimously that further study was needed to evaluate the potential association of hydroxyprogesterone caproate with increased risk of second trimester miscarriage and stillbirth.[10] A toxicology study in rhesus monkeys resulted in the death of all rhesus fetuses exposed to 1 and 10 times the human dose equivalent of hydroxyprogesterone caproate.[11] as of 2008, hydroxyprogesterone caproate was a category D progestin according to the FDA (that is, there is evidence of fetal harm). There is speculation that the castor oil in the hydroxyprogesterone caproate formulation may not be beneficial for pregnancy.[12][13] Of note, the above-mentioned NEJM study by Meirs et al. compares the effect of hydroxyprogesterone caproate (with the castor oil component) to castor oil injection as the placebo.
Pharmacology and pharmacokinetics
Hydroxyprogesterone caproate, unlike many other progestins, is very similar to natural progesterone both structurally and pharmacologically, and is a pure progestogen. However, relative to progesterone, hydroxyprogesterone caproate has improved pharmacokinetics, namely, a much longer duration with depot injection.
The caproate ester is not cleaved from hydroxyprogesterone caproate during metabolism, so it is not converted into 17α-hydroxyprogesterone, nor into progesterone.[14] Hydroxyprogesterone caproate is a much more potent progestogen relative to 17α-hydroxyprogesterone, but does not have as high of affinity for the progesterone receptor (PR) relative to progesterone.[14] However, despite this, it is more potent than progesterone in vivo, likely due to differences in the pharmacokinetics of the two compounds.[14] Hydroxyprogesterone caproate is not as potent as hydroxyprogesterone acetate.[14]
Hydroxyprogesterone caproate, unlike various other progestogens, is a pure progestogen,[15] and has no androgenic or glucocorticoidic properties,[16] nor any estrogenic effects.[15] Due to its lack of androgenic properties, similarly to progesterone, hydroxyprogesterone caproate does not have any teratogenic effects on the fetus, making it safe for use during pregnancy.[16] Regarding glucocorticoid activity, hydroxyprogesterone caproate has been found not to alter cortisol levels in humans even with extremely high dosages via intramuscular (i.m.) injection,[17] which is of relevance because drugs with significant glucocorticoid activity suppress cortisol levels (due to increased negative feedback on the hypothalamic-pituitary-adrenal axis). Hydroxyprogesterone caproate has been studied in humans at dosages as high as 5,000 mg per week via i.m. injection (in the treatment of endometrial cancer specifically, with safety and effectiveness observed).[18] Through activation of the PR, hydroxyprogesterone caproate has antigonadotropic effects.[19]
Hydroxyprogesterone caproate has a half-life of 7.8 days via intramuscular injection.[20]
Synthesis
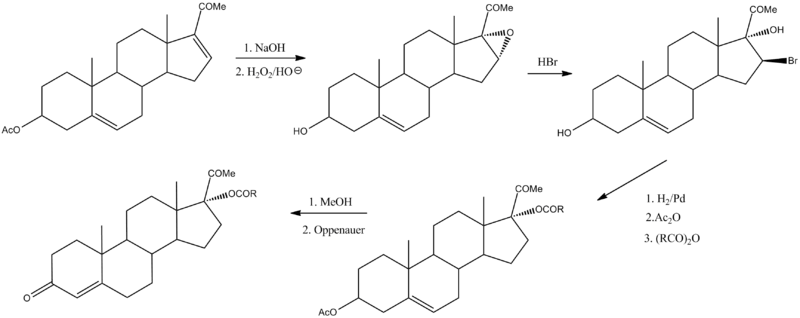
Hydroxyprogesterone caproate can be prepared by the following sequence:[21]
It is made from 16-dehydropregnenolone acetate (16-DPA),[22] product of the Marker degradation.
Makena
A 2011 decision by the USFDA was going to result in driving "up the [US] cost of an available medication from about $300 to $30,000 — about a 100-fold increase — with minimal added clinical benefit".[23] However, the USFDA said it would not go after compounding pharmacies that filled prescriptions, and KV Pharmaceutical announced a lower price.[23]
See also
Notes
- 1 2 3 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1.
- ↑ SMFM Clinical Guideline: Progesterone and preterm birth prevention: translating clinical trials data into clinical practice, AJOG May 2012
- ↑ Meirs et al. NEJM 2003
- ↑ Dodd JM, Flenady V, Cincotta R, Crowther CA; The Cochrane Database of Systematic Reviews 2006 Issue 1
- ↑ Keirse, MJNC; Progesterone (2004). "déjà vu" or "still to be seen"?.". Birth 31: 3.
- ↑ Johnson, JWC; Austin, KL; Jones, GS; Davis, GH; King, TM (1975). "Efficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of premature labor". NEJM 293 (14): 675. doi:10.1056/nejm197510022931401.
- ↑ Yemini, M; Borenstein, R; Dreazen; et al. (1985). "Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate". Am J Obstet Gynecol 151 (5): 574–7. doi:10.1016/0002-9378(85)90141-3.
- ↑ Meis PJ et al. Prevention of Recurrent Preterm Delivery by 17 Alpha-hydroxyprogesterone Caproate. NEJM, 2003: vol 348, no 24, pg 2379-2385.
- ↑ Keirse MJNC, Progestogen administration in pregnancy may prevent preterm delivery. Br J Obstet Gynecol 1990 February; 97:149.
- ↑ Advisory Committees: CDER 2006 Meeting Documents
- ↑ Hendrix AG, et al. Embriotoxicity of sex steroidal hormones in nonhuman primates: II. Hydroxyprogesterone caproate, estradiol valerate. Teratology 1987 February. 35 (1): 129.
- ↑ Duke University Medical Center, New England Journal of Medicine, correspondence, vol 349.
- ↑ Hauth, JC; Gilstrap, LC; Brekken, AL; Hauth, JM (1983). "The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population". Am J Obstet Gynecol 146 (2): 187.
- 1 2 3 4 Attardi BJ, Zeleznik A, Simhan H, Chiao JP, Mattison DR, Caritis SN (2007). "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". Am. J. Obstet. Gynecol. 197 (6): 599.e1–7. doi:10.1016/j.ajog.2007.05.024. PMC 2278032. PMID 18060946.
- 1 2 Geller, Jack (1965). "Treatment of Benign Prostatic Hypertrophy With Hydroxyprogesterone Caproate". JAMA 193 (2): 121. doi:10.1001/jama.1965.03090020035009. ISSN 0098-7484.
- 1 2 Meis PJ (2005). "17 hydroxyprogesterone for the prevention of preterm delivery". Obstet Gynecol 105 (5 Pt 1): 1128–35. doi:10.1097/01.AOG.0000160432.95395.8f. PMID 15863556.
- ↑ Onsrud, Mathias; Paus, Elisabeth; Haug, Egil; Ørstad, Kjell K (1985). "Intramuscular Administration of Hydroxyprogesterone Caproate in patients with Endometrial Carcinoma: Pharmacokinetics and effects on adrenal function". Acta Obstetricia et Gynecologica Scandinavica 64 (6): 519–523. doi:10.3109/00016348509156732. ISSN 0001-6349.
- ↑ Varga A, Henriksen E. Clinical and Histopathologic Evaluation of the Effect of 17-alpha-Hydroxyprogesterone-17-n-caproate on Endometrial Carcinoma. Obstetrics & Gynecology. December 1961. Volume 18. Issue 6. pp. 658-672.
- ↑ Benign Prostatic Hypertrophy. Springer Science & Business Media. 6 December 2012. pp. 266–. ISBN 978-1-4612-5476-8.
- ↑ "Hydroxyprogesterone Caproate". Drugs.com.
- ↑ Ringold, H. J.; Loken, B.; Rosenkraz, G.; Sondheimer, F. (1956). "Steroids. LXXIII. The Direct Oppenauer Oxidation of Steroidal Formate Esters. A New Synthesis of 17α-Hydroxyprogesterone". J. Amer. Chem. Soc. 78 (4): 816. doi:10.1021/ja01585a030.
- ↑ Goswami, A.; Kotoky, R.; Rastogi, R. C.; Ghosh, A. C. (2003). "A One-Pot Efficient Process for 16-Dehydropregnenolone Acetate". Organic Process Research & Development 7 (3): 306. doi:10.1021/op0200625.
- 1 2 Armstrong J (May 2011). "Unintended consequences — the cost of preventing preterm births after FDA approval of a branded version of 17OHP". N. Engl. J. Med. 364 (18): 1689–91. doi:10.1056/NEJMp1102796. PMID 21410391.
Sources
- FDA Reproductive Health Drugs Advisory Committee. August 29, 2006 Meeting to discuss NDA 21-945 Gestiva (Adeza Biomedical)
17α-hydroxyprogesterone caproate injection, 250 mg/mL, for the proposed indication: prevention of preterm delivery in women with a history of a prior preterm delivery. - Adeza Biomedical (October 23, 2006) Receives FDA Approvable Letter For Gestiva
- Adeza gets orphan drug designation for Gestiva (January 31, 2007)
- Cytyc Acquires Adeza Biomedical Corporation (April 3, 2007)
Adeza's name changed to Cytyc Prenatal Products Corp.
| ||||||||||||||||||||||||||||||||||||||