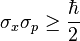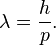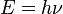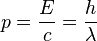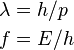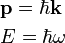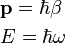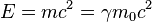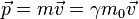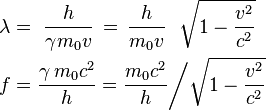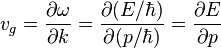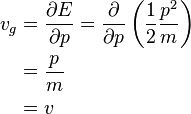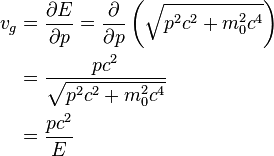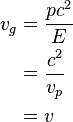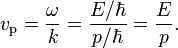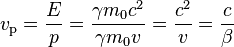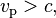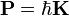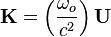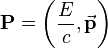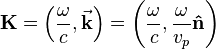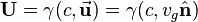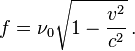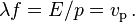Matter wave
All matter can exhibit wave-like behaviour. For example a beam of electrons can be diffracted just like a beam of light or a water wave. Matter waves are a central part of the theory of quantum mechanics, being an example of wave–particle duality. The concept that matter behaves like a wave is also referred to as the de Broglie hypothesis (/dəˈbrɔɪ/) due to having been proposed by Louis de Broglie in 1924.[1] Matter waves are often referred to as de Broglie waves.
The de Broglie wavelength is the wavelength, λ, associated with a massive particle and is related to its momentum, p, through the Planck constant, h:
Wave-like behaviour of matter was first experimentally demonstrated in the Davisson–Germer experiment using electrons, and it has also been confirmed for other elementary particles, neutral atoms and even molecules. The wave-like behaviour of matter is crucial to the modern theory of atomic structure and particle physics.
Historical context
At the end of the 19th century, light was thought to consist of waves of electromagnetic fields which propagated according to Maxwell’s equations, while matter was thought to consist of localized particles (See history of wave and particle viewpoints). In 1900, this division was exposed to doubt, when, investigating the theory of black body thermal radiation, Max Planck proposed that light is emitted in discrete quanta of energy. It was thoroughly challenged in 1905. Extending Planck's investigation in several ways, including its connection with the photoelectric effect, Albert Einstein proposed that light is also propagated and absorbed in quanta. Light quanta are now called photons. These quanta would have an energy given by the Planck–Einstein relation:
and a momentum
where ν (lowercase Greek letter nu) and λ (lowercase Greek letter lambda) denote the frequency and wavelength of the light, c the speed of light, and h Planck’s constant.[2] In the modern convention, frequency is symbolized by f as is done in the rest of this article. Einstein’s postulate was confirmed experimentally by Robert Millikan and Arthur Compton over the next two decades.
The de Broglie hypothesis

De Broglie, in his 1924 PhD thesis, proposed that just as light has both wave-like and particle-like properties, electrons also have wave-like properties. By rearranging the momentum equation stated in the above section, we find a relationship between the wavelength, λ associated with an electron and its momentum, p, through the Planck constant, h:[3]
The relationship is now known to hold for all types of matter: all matter exhibits properties of both particles and waves.
| “ | When I conceived the first basic ideas of wave mechanics in 1923–24, I was guided by the aim to perform a real physical synthesis, valid for all particles, of the coexistence of the wave and of the corpuscular aspects that Einstein had introduced for photons in his theory of light quanta in 1905. | ” | |
| — De Broglie[4] | |||
In 1926, Erwin Schrödinger published an equation describing how a matter wave should evolve—the matter wave analogue of Maxwell’s equations—and used it to derive the energy spectrum of hydrogen.
Experimental confirmation

Matter waves were first experimentally confirmed to occur in the Davisson-Germer experiment for electrons, and the de Broglie hypothesis has been confirmed for other elementary particles. Furthermore, neutral atoms and even molecules have been shown to be wave-like.
Electrons
In 1927 at Bell Labs, Clinton Davisson and Lester Germer fired slow-moving electrons at a crystalline nickel target. The angular dependence of the diffracted electron intensity was measured, and was determined to have the same diffraction pattern as those predicted by Bragg for x-rays. Before the acceptance of the de Broglie hypothesis, diffraction was a property that was thought to be only exhibited by waves. Therefore, the presence of any diffraction effects by matter demonstrated the wave-like nature of matter. When the de Broglie wavelength was inserted into the Bragg condition, the observed diffraction pattern was predicted, thereby experimentally confirming the de Broglie hypothesis for electrons.[5]
This was a pivotal result in the development of quantum mechanics. Just as the photoelectric effect demonstrated the particle nature of light, the Davisson–Germer experiment showed the wave-nature of matter, and completed the theory of wave-particle duality. For physicists this idea was important because it meant that not only could any particle exhibit wave characteristics, but that one could use wave equations to describe phenomena in matter if one used the de Broglie wavelength.
Neutral atoms
Experiments with Fresnel diffraction[6] and an atomic mirror for specular reflection[7][8] of neutral atoms confirm the application of the de Broglie hypothesis to atoms, i.e. the existence of atomic waves which undergo diffraction, interference and allow quantum reflection by the tails of the attractive potential.[9] Advances in laser cooling have allowed cooling of neutral atoms down to nanokelvin temperatures. At these temperatures, the thermal de Broglie wavelengths come into the micrometre range. Using Bragg diffraction of atoms and a Ramsey interferometry technique, the de Broglie wavelength of cold sodium atoms was explicitly measured and found to be consistent with the temperature measured by a different method.[10]
This effect has been used to demonstrate atomic holography, and it may allow the construction of an atom probe imaging system with nanometer resolution.[11][12] The description of these phenomena is based on the wave properties of neutral atoms, confirming the de Broglie hypothesis.
The effect has also been used to explain the spatial version of the quantum Zeno effect, in which an otherwise unstable object may be stabilised by rapidly-repeated observations.[8]
Molecules
Recent experiments even confirm the relations for molecules and even macromolecules that otherwise might be supposed too large to undergo quantum mechanical effects. In 1999, a research team in Vienna demonstrated diffraction for molecules as large as fullerenes.[13] The researchers calculated a De Broglie wavelength of the most probable C60 velocity as 2.5 pm. More recent experiments prove the quantum nature of molecules with a mass up to 6910 amu.[14]
de Broglie relations
The de Broglie equations relate the wavelength λ to the momentum p, and frequency f to the total energy E of a particle:[15]
where h is Planck's constant. The equations can also be written as
or [16]
where 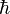 is the reduced Planck's constant,
is the reduced Planck's constant, 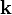 is the wave vector,
is the wave vector, 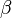 is the phase constant,and
is the phase constant,and 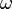 is the angular frequency.
is the angular frequency.
In each pair, the second equation is also referred to as the Planck-Einstein relation, since it was also proposed by Planck and Einstein.
Special relativity
Using two formulas from special relativity, one for the relativistic momentum and one for the energy
allows the equations to be written as
where 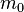 denotes the particle's rest mass,
denotes the particle's rest mass, 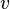 its velocity,
its velocity, 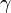 the Lorentz factor, and
the Lorentz factor, and  the speed of light in a vacuum.[17][18][19] See below for details of the derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity (equal to the product of the particle's frequency and its wavelength). In the case of a non-dispersive medium, they happen to be equal, but otherwise they are not.
the speed of light in a vacuum.[17][18][19] See below for details of the derivation of the de Broglie relations. Group velocity (equal to the particle's speed) should not be confused with phase velocity (equal to the product of the particle's frequency and its wavelength). In the case of a non-dispersive medium, they happen to be equal, but otherwise they are not.
Group velocity
Albert Einstein first explained the wave–particle duality of light in 1905. Louis de Broglie hypothesized that any particle should also exhibit such a duality. The velocity of a particle, he concluded, should always equal the group velocity of the corresponding wave. The magnitude of the group velocity is equal to the particle's speed.
Both in relativistic and non-relativistic quantum physics, we can identify the group velocity of a particle's wave function with the particle velocity. Quantum mechanics has very accurately demonstrated this hypothesis, and the relation has been shown explicitly for particles as large as molecules.
De Broglie deduced that if the duality equations already known for light were the same for any particle, then his hypothesis would hold. This means that
where E is the total energy of the particle, p is its momentum, ħ is the reduced Planck constant. For a free non-relativistic particle it follows that
where m is the mass of the particle and v its velocity.
Also in special relativity we find that
where 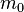 is the rest mass of the particle and
is the rest mass of the particle and  is the speed of light in a vacuum. But (see below), using that the phase velocity is
is the speed of light in a vacuum. But (see below), using that the phase velocity is 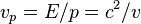 , therefore
, therefore
where v is the velocity of the particle regardless of wave behavior.
Phase velocity
In quantum mechanics, particles also behave as waves with complex phases. The phase velocity is equal to the product of the frequency multiplied by the wavelength.
By the de Broglie hypothesis, we see that
Using relativistic relations for energy and momentum, we have
where E is the total energy of the particle (i.e. rest energy plus kinetic energy in kinematic sense), p the momentum, 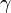 the Lorentz factor, c the speed of light, and β the speed as a fraction of c. The variable v can either be taken to be the speed of the particle or the group velocity of the corresponding matter wave. Since the particle speed
the Lorentz factor, c the speed of light, and β the speed as a fraction of c. The variable v can either be taken to be the speed of the particle or the group velocity of the corresponding matter wave. Since the particle speed 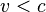 for any particle that has mass (according to special relativity), the phase velocity of matter waves always exceeds c, i.e.
for any particle that has mass (according to special relativity), the phase velocity of matter waves always exceeds c, i.e.
and as we can see, it approaches c when the particle speed is in the relativistic range. The superluminal phase velocity does not violate special relativity, because phase propagation carries no energy. See the article on Dispersion (optics) for details.
Four-vectors
Using 4-Vectors, the De Broglie relations form a single equation:
which is frame-independent.
Likewise, the relation between group/particle velocity and phase velocity is given in frame-independent form by:
where
Interpretations
The physical reality underlying de Broglie waves is a subject of ongoing debate. Some theories treat either the particle or the wave aspect as its fundamental nature, seeking to explain the other as an emergent property. Some, such as the hidden variable theory, treat the wave and the particle as distinct entities. Yet others propose some intermediate entity that is neither quite wave nor quite particle but only appears as such when we measure one or the other property. The Copenhagen interpretation states that the nature of the underlying reality is unknowable and beyond the bounds of scientific enquiry.
Schrödinger's quantum mechanical waves are conceptually different from ordinary physical waves such as water or sound. Ordinary physical waves are characterized by undulating real-number 'displacements' of dimensioned physical variables at each point of ordinary physical space at each instant of time. Schrödinger's "waves" are characterized by the undulating value of a dimensionless complex number at each point of an abstract multi-dimensional space, for example of configuration space.
At the Fifth Solvay Conference in 1927, Max Born and Werner Heisenberg reported as follows
| “ | If one wishes to calculate the probabilities of excitation and ionization of atoms [M. Born, Zur Quantenmechanik der Stossvorgange, Z. f. Phys., 37 (1926), 863; [Quantenmechanik der Stossvorgange], ibid., 38 (1926), 803] then one must introduce the coordinates of the atomic electrons as variables on an equal footing with those of the colliding electron. The waves then propagate no longer in three-dimensional space but in multi-dimensional configuration space. From this one sees that the quantum mechanical waves are indeed something quite different from the light waves of the classical theory.[20] | ” |
At the same conference, Erwin Schrödinger reported likewise.
| “ | Under [the name 'wave mechanics',] at present two theories are being carried on, which are indeed closely related but not identical. The first, which follows on directly from the famous doctoral thesis by L. de Broglie, concerns waves in three-dimensional space. Because of the strictly relativistic treatment that is adopted in this version from the outset, we shall refer to it as the four-dimensional wave mechanics. The other theory is more remote from Mr de Broglie's original ideas, insofar as it is based on a wave-like process in the space of position coordinates (q-space) of an arbitrary mechanical system.[Long footnote about manuscript not copied here.] We shall therefore call it the multi-dimensional wave mechanics. Of course this use of the q-space is to be seen only as a mathematical tool, as it is often applied also in the old mechanics; ultimately, in this version also, the process to be described is one in space and time. In truth, however, a complete unification of the two conceptions has not yet been achieved. Anything over and above the motion of a single electron could be treated so far only in the multi-dimensional version; also, this is the one that provides the mathematical solution to the problems posed by the Heisenberg-Born matrix mechanics.[21] | ” |
In 1955, Heisenberg reiterated this.
| “ | An important step forward was made by the work of Born [Z. Phys., 37: 863, 1926 and 38: 803, 1926] in the summer of 1926. In this work, the wave in configuration space was interpreted as a probability wave, in order to explain collision processes on Schrödinger's theory. This hypothesis contained two important new features in comparison with that of Bohr, Kramers and Slater. The first of these was the assertion that, in considering "probability waves", we are concerned with processes not in ordinary three-dimensional space, but in an abstract configuration space (a fact which is, unfortunately, sometimes overlooked even today); the second was the recognition that the probability wave is related to an individual process.[22] | ” |
It is mentioned above that the "displaced quantity" of the Schrödinger wave has values that are dimensionless complex numbers. One may ask what is the physical meaning of those numbers. According to Heisenberg, rather than being of some ordinary physical quantity such as for example Maxwell's electric field intensity, or for example mass density, the Schrödinger-wave packet's "displaced quantity" is probability amplitude. He wrote that instead of using the term 'wave packet', it is preferable to speak of a probability packet. [23] The probability amplitude supports calculation of probability of location or momentum of discrete particles. Heisenberg recites Duane's account of particle diffraction by probabilistic quantal translation momentum transfer, which allows, for example in Young's two-slit experiment, each diffracted particle probabilistically to pass discretely through a particular slit.[24] Thus one does not need necessarily think of the matter wave, as it were, as 'composed of smeared matter'.
These ideas may be expressed in ordinary language as follows. In the account of ordinary physical waves, a 'point' refers to a position in ordinary physical space at an instant of time, at which there is specified a 'displacement' of some physical quantity. But in the account of quantum mechanics, a 'point' refers to a configuration of the system at an instant of time, every particle of the system being in a sense present in every 'point' of configuration space, each particle at such a 'point' being located possibly at a different position in ordinary physical space. There is no explicit definite indication that, at an instant, this particle is 'here' and that particle is 'there' in some separate 'location' in configuration space. This conceptual difference entails that, in contrast to de Broglie's pre-quantum mechanical wave description, the quantum mechanical probability packet description does not directly and explicitly express the Aristotelian idea, referred to by Newton, that causal efficacy propagates through ordinary space by contact, nor the Einsteinian idea that such propagation is no faster than light. In contrast, these ideas are so expressed in the classical wave account, through the Green's function, though it is inadequate for the observed quantal phenomena. The physical reasoning for this was first recognized by Einstein.[25][26]
De Broglie's phase wave and periodic phenomenon
De Broglie's thesis started from the hypothesis, "that to each portion of energy with a proper mass m0 one may associate a periodic phenomenon of the frequency ν0 , such that one finds: hν0 = m0c2. The frequency ν0 is to be measured, of course, in the rest frame of the energy packet. This hypothesis is the basis of our theory."[27][28][29][30][31][32]
De Broglie followed his initial hypothesis of a periodic phenomenon, with frequency ν0 , associated with the energy packet. He used the special theory of relativity to find, in the frame of the observer of the electron energy packet that is moving with velocity 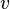 , that its frequency was apparently reduced to
, that its frequency was apparently reduced to
Then
using the same notation as above. The quantity 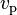 is the velocity of what de Broglie called the "phase wave". Its wavelength is
is the velocity of what de Broglie called the "phase wave". Its wavelength is  and frequency
and frequency 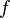 . De Broglie reasoned that his hypothetical intrinsic particle periodic phenomenon is in phase with that phase wave. This was his basic matter wave conception. He noted, as above, that
. De Broglie reasoned that his hypothetical intrinsic particle periodic phenomenon is in phase with that phase wave. This was his basic matter wave conception. He noted, as above, that 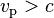 , and the phase wave does not transfer energy.[29][33]
, and the phase wave does not transfer energy.[29][33]
While the concept of waves being associated with matter is correct, de Broglie did not leap directly to the final understanding of quantum mechanics with no missteps. There are conceptual problems with the approach that de Broglie took in his thesis that he was not able to resolve, despite trying a number of different fundamental hypotheses in different papers published while working on, and shortly after publishing, his thesis.[30][34] These difficulties were resolved by Erwin Schrödinger, who developed the wave mechanics approach, starting from a somewhat different basic hypothesis.
See also
- Bohr model
- Faraday wave
- Kapitsa–Dirac effect
- Matter wave clock
- Schrödinger equation
- Theoretical and experimental justification for the Schrödinger equation
- Thermal de Broglie wavelength
- De Broglie–Bohm theory
References
- ↑ Feynman, R.; QED the Strange Theory of Light and matter, Penguin 1990 Edition, page 84.
- ↑ Einstein, A. (1917). Zur Quantentheorie der Strahlung, Physicalische Zeitschrift 18: 121–128. Translated in ter Haar, D. (1967). The Old Quantum Theory. Pergamon Press. pp. 167–183. LCCN 66029628.
- ↑ J. P. McEvoy & Oscar Zarate (2004). Introducing Quantum Theory. Totem Books. pp. 110–114. ISBN 1-84046-577-8.
- ↑ Louis de Broglie "The Reinterpretation of Wave Mechanics" Foundations of Physics, Vol. 1 No. 1 (1970)
- ↑ Mauro Dardo, Nobel Laureates and Twentieth-Century Physics, Cambridge University Press 2004, pp. 156–157
- ↑ R.B.Doak; R.E.Grisenti; S.Rehbein; G.Schmahl; J.P.Toennies; Ch. Wöll (1999). "Towards Realization of an Atomic de Broglie Microscope: Helium Atom Focusing Using Fresnel Zone Plates". Physical Review Letters 83 (21): 4229–4232. Bibcode:1999PhRvL..83.4229D. doi:10.1103/PhysRevLett.83.4229.
- ↑ F. Shimizu (2000). "Specular Reflection of Very Slow Metastable Neon Atoms from a Solid Surface". Physical Review Letters 86 (6): 987–990. Bibcode:2001PhRvL..86..987S. doi:10.1103/PhysRevLett.86.987. PMID 11177991.
- 1 2 D. Kouznetsov; H. Oberst (2005). "Reflection of Waves from a Ridged Surface and the Zeno Effect". Optical Review 12 (5): 1605–1623. Bibcode:2005OptRv..12..363K. doi:10.1007/s10043-005-0363-9.
- ↑ H.Friedrich; G.Jacoby; C.G.Meister (2002). "quantum reflection by Casimir–van der Waals potential tails". Physical Review A 65 (3): 032902. Bibcode:2002PhRvA..65c2902F. doi:10.1103/PhysRevA.65.032902.
- ↑ Pierre Cladé; Changhyun Ryu; Anand Ramanathan; Kristian Helmerson; William D. Phillips (2008). "Observation of a 2D Bose Gas: From thermal to quasi-condensate to superfluid". arXiv:0805.3519.
- ↑ Shimizu; J.Fujita (2002). "Reflection-Type Hologram for Atoms". Physical Review Letters 88 (12): 123201. Bibcode:2002PhRvL..88l3201S. doi:10.1103/PhysRevLett.88.123201. PMID 11909457.
- ↑ D. Kouznetsov; H. Oberst; K. Shimizu; A. Neumann; Y. Kuznetsova; J.-F. Bisson; K. Ueda; S. R. J. Brueck (2006). "Ridged atomic mirrors and atomic nanoscope". Journal of Physics B 39 (7): 1605–1623. Bibcode:2006JPhB...39.1605K. doi:10.1088/0953-4075/39/7/005.
- ↑ Arndt, M.; O. Nairz; J. Voss-Andreae; C. Keller; G. van der Zouw; A. Zeilinger (14 October 1999). "Wave-particle duality of C60". Nature 401 (6754): 680–682. Bibcode:1999Natur.401..680A. doi:10.1038/44348. PMID 18494170.
- ↑ Gerlich, S.; S. Eibenberger; M. Tomandl; S. Nimmrichter; K. Hornberger; P. J. Fagan; J. Tüxen; M. Mayor & M. Arndt (5 April 2011). "Quantum interference of large organic molecules". Nature Communications 2 (263): 263–. Bibcode:2011NatCo...2E.263G. doi:10.1038/ncomms1263. PMC 3104521. PMID 21468015.
- ↑ Resnick, R.; Eisberg, R. (1985). Quantum Physics of Atoms, Molecules, Solids, Nuclei and Particles (2nd ed.). New York: John Wiley & Sons. ISBN 0-471-87373-X.
- ↑ Z.Y.Wang (2016). "Generalized momentum equation of quantum mechanics". Optical and Quantum Electronics 48 (2). doi:10.1007/s11082-015-0261-8.
- ↑ Holden, Alan (1971). Stationary states. New York: Oxford University Press. ISBN 0-19-501497-9.
- ↑ Williams, W.S.C. (2002). Introducing Special Relativity, Taylor & Francis, London, ISBN 0-415-27761-2, p. 192.
- ↑ de Broglie, L. (1970). The reinterpretation of wave mechanics, Foundations of Physics 1(1): 5–15, p. 9.
- ↑ Born, M., Heisenberg, W. (1928). Quantum mechanics, pp. 143–181 of Électrons et Photons: Rapports et Discussions du Cinquième Conseil de Physique, tenu à Bruxelles du 24 au 29 Octobre 1927, sous les Auspices de l'Institut International de Physique Solvay, Gauthier-Villars, Paris, p. 166; this translation at p. 425 of Bacciagaluppi, G., Valentini, A. (2009), Quantum Theory at the Crossroads: Reconsidering the 1927 Solvay Conference, Cambridge University Press, Cambridge UK, ISBN 978-0-521-81421-8.
- ↑ Schrödinger, E. (1928). Wave mechanics, pp. 185–206 of Électrons et Photons: Rapports et Discussions du Cinquième Conseil de Physique, tenu à Bruxelles du 24 au 29 Octobre 1927, sous les Auspices de l'Institut International de Physique Solvay, Gauthier-Villars, Paris, pp. 185–186; this translation at p. 447 of Bacciagaluppi, G., Valentini, A. (2009), Quantum Theory at the Crossroads: Reconsidering the 1927 Solvay Conference, Cambridge University Press, Cambridge UK, ISBN 978-0-521-81421-8.
- ↑ Heisenberg, W. (1955). The development of the interpretation of the quantum theory, pp. 12–29, in Niels Bohr and the Development of Physics: Essays dedicated to Niels Bohr on the occasion of his seventieth birthday, edited by W. Pauli, with the assistance of L. Rosenfeld and V. Weisskopf, Pergamon Press, London, p. 13.
- ↑ Heisenberg, W. (1927). Über den anschlaulichen Inhalt der quantentheoretischen Kinematik und Mechanik, Z. Phys. 43: 172–198, translated by eds. Wheeler, J.A., Zurek, W.H. (1983), at pp. 62–84 of Quantum Theory and Measurement, Princeton University Press, Princeton NJ, p. 73. Also translated as 'The actual content of quantum theoretical kinematics and mechanics' here
- ↑ Heisenberg, W. (1930). The Physical Principles of the Quantum Theory, translated by C. Eckart, F. C. Hoyt, University of Chicago Press, Chicago IL, pp. 77–78.
- ↑ Fine, A. (1986). The Shaky Game: Einstein Realism and the Quantum Theory, University of Chicago, Chicago, ISBN 0-226-24946-8
- ↑ Howard, D. (1990). "Nicht sein kann was nicht sein darf", or the prehistory of the EPR, 1909–1935; Einstein's early worries about the quantum mechanics of composite systems, pp. 61–112 in Sixty-two Years of Uncertainty: Historical Philosophical and Physical Inquiries into the Foundations of Quantum Mechanics, edited by A.I. Miller, Plenum Press, New York, ISBN 978-1-4684-8773-2.
- ↑ de Broglie, L. (1923). Waves and quanta, Nature 112: 540.
- ↑ de Broglie, L. (1924). Thesis, p. 8 of Kracklauer's translation.
- 1 2 Medicus, H.A. (1974). Fifty years of matter waves, Physics Today 27(2): 38–45.
- 1 2 MacKinnon, E. (1976). De Broglie's thesis: a critical retrospective, Am. J. Phys. 44: 1047–1055.
- ↑ Espinosa, J.M. (1982). Physical properties of de Broglie's phase waves, Am. J. Phys. 50: 357–362.
- ↑ Brown, H.R., Martins, R.deA. (1984). De Broglie's relativistic phase waves and wave groups, Am. J. Phys. 52: 1130–1140.
- ↑ Bacciagaluppi, G., Valentini, A. (2009). Quantum Theory at the Crossroads: Reconsidering the 1927 Solvay Conference, Cambridge University Press, Cambridge UK, ISBN 978-0-521-81421-8, pp. 30–88.
- ↑ Martins, Roberto de Andrade (2010). "Louis de Broglie's Struggle with the Wave-Particle Dualism, 1923-1925". Quantum History Project, Fritz Haber Institute of the Max Planck Society and the Max Planck Institute for the History of Science. Retrieved 2015-01-03.
Further reading
- L. de Broglie, Recherches sur la théorie des quanta (Researches on the quantum theory), Thesis (Paris), 1924; L. de Broglie, Ann. Phys. (Paris) 3, 22 (1925). English translation by A.F. Kracklauer. And here.
- Broglie, Louis de, The wave nature of the electron Nobel Lecture, 12, 1929
- Tipler, Paul A. and Ralph A. Llewellyn (2003). Modern Physics. 4th ed. New York; W. H. Freeman and Co. ISBN 0-7167-4345-0. pp. 203–4, 222–3, 236.
- Zumdahl, Steven S. (2005). Chemical Principles (5th ed.). Boston: Houghton Mifflin. ISBN 0-618-37206-7.
- An extensive review article "Optics and interferometry with atoms and molecules" appeared in July 2009: http://www.atomwave.org/rmparticle/RMPLAO.pdf.
- "Scientific Papers Presented to Max Born on his retirement from the Tait Chair of Natural Philosophy in the University of Edinburgh", 1953 (Oliver and Boyd)
External links
- Bowley, Roger. "de Broglie Waves". Sixty Symbols. Brady Haran for the University of Nottingham.