Dakin–West reaction
The Dakin–West reaction is a chemical reaction that transforms an amino-acid into a keto-amide using an acid anhydride and a base, typically pyridine. It is named for Henry Drysdale Dakin (1880–1952) and Randolph West (1890–1949). Of special note, the keto-amide product is always racemic.

With pyridine as a base and solvent, refluxing conditions are required. However, with the addition of 4-dimethylaminopyridine (DMAP) as a catalyst, the reaction can take place at room temperature.
With some acids, this reaction can take place even in the absence of an α-amino group.
This reaction should not be confused with the Dakin reaction.
Reaction mechanism
The reaction mechanism involves the acylation and activation of the acid 1 to the mixed anhydride 3. The amide will serve as a nucleophile for the cyclization forming the azlactone 4. Deprotonation and acylation of the azlactone forms the key carbon-carbon bond. Subsequent ring-opening of 6 and decarboxylation give the final keto-amide product.

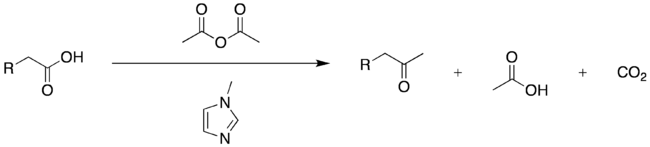
General ketone synthesis
Modern variations on the Dakin–West reaction permit many enolizable carboxylic acids – not merely amino acids – to be converted to their corresponding methyl ketones. For example, β-aryl carboxylic acids can be efficiently converted to β-aryl ketones by treatment of an acetic anhydride solution of the acid with catalytic N-methylimidazole. This reactivity is attributed in part to generation of acetylimidazolium, a powerful cationic acetylating agent, in situ.
See Also
- Robinson–Gabriel synthesis - A process for converting the keto-amide products of this reaction into oxazoles
References
- ^ (a) Dakin, H. D.; West, R. J. Biol. Chem. 1928, 78, 91. (b) Dakin, H. D.; West, R. J. Biol. Chem. 1928, 78, 745. (c) Dakin, H. D.; West, R. J. Biol. Chem. 1928, 78, 757.
- ^ Wiley, R. H. J. Org. Chem. 1947, 12, 43–46. (doi:10.1021/jo01165a006)
- ^ Buchanan, G. L. Chem. Soc. Rev. 1988, 17, 91–109. (Review)
- ^ Wiley, R. H.; Borum, O. H. Organic Syntheses, Coll. Vol. 4, p. 5 (1963); Vol. 33, p. 1 (1953). (Article)
- ^ Hoefle, G.; Steglich, W.; Vorbrueggen, H. Angew. Chem. Int. Ed. Engl. 1978, 17, 569.
- ^ Knorr, R.; Huisgen, R. Ber. 1970, 103, 2598.
- ^ Allinger, N. et al. J. Org. Chem. 1974, 39, 1730. (doi:10.1021/jo00925a029)
- ^ Tran, K.; Bickar, D. J. Org. Chem. 2006, 71, 6640–6643. (doi:10.1021/jo0607966)