Thymidine triphosphate
 | |
 | |
| Names | |
|---|---|
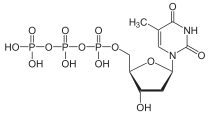
| IUPAC name
[(2R,3S,5R)-3-hydroxy-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methyl (hydroxy-phosphonooxyphosphoryl) hydrogen phosphate | |
| Other names
dTTP, 2'-deoxythymidine triphosphate | |
| Identifiers | |
| 365-08-2 | |
| ChEMBL | ChEMBL363559 |
| ChemSpider | 58493 |
| 5505 | |
| Jmol interactive 3D | Image |
| MeSH | thymidine+5'-triphosphate |
| PubChem | 64968 |
| |
| Properties | |
| C10H17N2O14P3 | |
| Molar mass | 482.168 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Deoxythymidine triphosphate (dTTP) is one of the four nucleoside triphosphates that are used in the in vivo synthesis of DNA. Unlike the other deoxyribonucleotide triphosphates, thymidine triphosphate does not contain the "deoxy" prefix in its name.[1] The corresponding ribonucleoside triphosphate is called uridine triphosphate.
It can be used by DNA ligase to create overlapping "sticky ends" so that protruding ends of opened microbial plasmids may be closed up.
References
| ||||||||||||||||||||||||||||||||||||||||||||||||||
This article is issued from Wikipedia - version of the Friday, September 04, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.