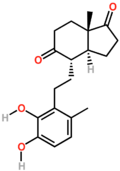
DHSA
 | |
| Names | |
|---|---|
| IUPAC name
3,4-Dihydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione | |
| Identifiers | |
| ChEBI | CHEBI:15896 |
| ChemSpider | 389410 |
| DrugBank | DB08542 |
| Jmol interactive 3D | Image |
| PubChem | 440483 |
| |
| |
| Properties | |
| C19H24O4 | |
| Molar mass | 316.39146 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
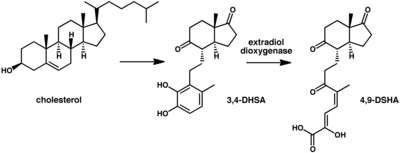
3,4-DHSA is an organic compound which is the intermediate product of the metabolism of cholesterol, by the bacteria most commonly responsible for tuberculosis (Mycobacterium tuberculosis).[1] 3,4-DHSA is an acronym for 3,4-dihydroxy-9,10-seco-androst-1,3,5(10)-triene-9,17-dione, the official name of this substance. It is classified as a secosteroid, since one of the four rings of cholesterol from which it is derived is broken.
3,4-DHSA is a catecholic intermediate (a compound containing an aromatic ring with two adjacent hydroxyl groups) produced by M. tuberculosis during the break down of cholesterol.[1] 3,4-DHSA is also produced by other bacteria such as Comamonas testosteroni.[2][3]
A particular type of enzyme known as extradiol dioxygenase is responsible for the oxidation and ring opening of 3,4-DHSA to 4,9-DSHA (see metabolic scheme below). M. tuberculosis bacteria that are deficient in this enzyme are less lethal than wild-type bacteria. 3,4-DHSA itself appears to be toxic to the bacteria while the breakdown products of 3,4-DHSA can be used as energy source by the bacteria. Hence blocking the oxidation of 3,4-DHSA by the extradiol dioxygenase enzyme may be useful in the treatment of tuberculosis.[1]
A crystal structure of DHSA in complex with M. tuberculosis iron-dependent extradiol dioxygenase has been determined.[1]
References
- 1 2 3 4 5 PDB: 2ZI8; Yam KC, D'Angelo I, Kalscheuer R, Zhu H, Wang JX, Snieckus V, Ly LH, Converse PJ, Jacobs WR, Strynadka N, Eltis LD (March 2009). Ramakrishnan, Lalita, ed. "Studies of a Ring-Cleaving Dioxygenase Illuminate the Role of Cholesterol Metabolism in the Pathogenesis of Mycobacterium tuberculosis". PLoS Pathog. 5 (3): e1000344. doi:10.1371/journal.ppat.1000344. PMC 2652662. PMID 19300498.
- ↑ Horinouchi M, Kurita T, Yamamoto T, Hatori E, Hayashi T, Kudo T (November 2004). "Steroid degradation gene cluster of Comamonas testosteroni consisting of 18 putative genes from meta-cleavage enzyme gene tesB to regulator gene tesR". Biochem. Biophys. Res. Commun. 324 (2): 597–604. doi:10.1016/j.bbrc.2004.09.096. PMID 15474469.
- ↑ Horinouchi M, Hayashi T, Kudo T (October 2004). "The genes encoding the hydroxylase of 3-hydroxy-9,10-secoandrosta-1,3,5(10)-triene-9,17-dione in steroid degradation in Comamonas testosteroni TA441". J. Steroid Biochem. Mol. Biol. 92 (3): 143–54. doi:10.1016/j.jsbmb.2004.09.002. PMID 15555908.