Cytochrome c oxidase
| Cytochrome c oxidase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Identifiers | |||||||||
| EC number | 1.9.3.1 | ||||||||
| CAS number | 9001-16-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
The enzyme cytochrome c oxidase, or Complex IV (EC 1.9.3.1), is a large transmembrane protein complex found in bacteria and the mitochondrion of eukaryotes.
It is the last enzyme in the respiratory electron transport chain of mitochondria or bacteria located in the mitochondrial or bacterial membrane. It receives an electron from each of four cytochrome c molecules, and transfers them to one oxygen molecule, converting molecular oxygen to two molecules of water. In the process, it binds four protons from the inner aqueous phase to make water, and in addition translocates four protons across the membrane, helping to establish a transmembrane difference of proton electrochemical potential that the ATP synthase then uses to synthesize ATP.[1][2]
Structure
The complex is a large integral membrane protein composed of several metal prosthetic sites and 14 [3] protein subunits in mammals. In mammals, eleven subunits are nuclear in origin, and three are synthesized in the mitochondria. The complex contains two hemes, a cytochrome a and cytochrome a3, and two copper centers, the CuA and CuB centers.[4] In fact, the cytochrome a3 and CuB form a binuclear center that is the site of oxygen reduction. Cytochrome c, which is reduced by the preceding component of the respiratory chain (cytochrome bc1 complex, complex III), docks near the CuA binuclear center and passes an electron to it, being oxidized back to cytochrome c containing Fe3+. The reduced CuA binuclear center now passes an electron on to cytochrome a, which in turn passes an electron on to the cytochrome a3-CuB binuclear center. The two metal ions in this binuclear center are 4.5 Å apart and coordinate a hydroxide ion in the fully oxidized state.
Crystallographic studies of cytochrome c oxidase show an unusual post-translational modification, linking C6 of Tyr(244) and the ε-N of His(240) (bovine enzyme numbering). It plays a vital role in enabling the cytochrome a3- CuB binuclear center to accept four electrons in reducing molecular oxygen to water. The mechanism of reduction was formerly thought to involve a peroxide intermediate, which was believed to lead to superoxide production. However, the currently accepted mechanism involves a rapid four-electron reduction involving immediate oxygen-oxygen bond cleavage, avoiding any intermediate likely to form superoxide.[5]
Assembly
The site of assembly is believed to occur near TOM/TIM, where complex intermediates are accessible to bind with subunits imported from cytosol. Hemes and cofactors are inserted into subunits I & II. Subunits I and IV initiate assembly. Other subunits may form sub-complex intermediates that later on, bind to others sub-complexes to form holo COX. In post-assembly modifications, the enzyme is dimerized, which is required for active/efficient enzyme action.[6][7]
Complex subunits
Conserved subunits of the cytochrome oxidase c complex:[8][9]
| No. | Subunit name | Human protein | Protein description from UniProt | Pfam family with Human protein |
|---|---|---|---|---|
| 1 | Cox1 | COX1_HUMAN | Cytochrome c oxidase subunit 1 | Pfam PF00115 |
| 2 | Cox2 | COX2_HUMAN | Cytochrome c oxidase subunit 2 | Pfam PF02790, Pfam PF00116 |
| 3 | Cox3 | COX3_HUMAN | Cytochrome c oxidase subunit 3 | Pfam PF00510 |
| 4 | Cox4i1 | COX41_HUMAN | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | Pfam PF02936 |
| 5 | Cox4a2 | COX42_HUMAN | Cytochrome c oxidase subunit 4 isoform 2, mitochondrial | Pfam PF02936 |
| 6 | Cox5a | COX5A_HUMAN | Cytochrome c oxidase subunit 5A, mitochondrial | Pfam PF02284 |
| 7 | Cox5b | COX5B_HUMAN | Cytochrome c oxidase subunit 5B, mitochondrial | Pfam PF01215 |
| 8 | Cox6a1 | CX6A1_HUMAN | Cytochrome c oxidase subunit 6A1, mitochondrial | Pfam PF02046 |
| 9 | Cox6a2 | CX6A2_HUMAN | Cytochrome c oxidase subunit 6A2, mitochondrial | Pfam PF02046 |
| 10 | Cox6b1 | CX6B1_HUMAN | Cytochrome c oxidase subunit 6B1 | Pfam PF02297 |
| 11 | Cox6b2 | CX6B2_HUMAN | Cytochrome c oxidase subunit 6B2 | Pfam PF02297 |
| 12 | Cox6c | COX6C_HUMAN | Cytochrome c oxidase subunit 6C | Pfam PF02937 |
| 13 | Cox7a1 | CX7A1_HUMAN | Cytochrome c oxidase subunit 7A1, mitochondrial | Pfam PF02238 |
| 14 | Cox7a2 | CX7A2_HUMAN | Cytochrome c oxidase subunit 7A2, mitochondrial | Pfam PF02238 |
| 15 | Cox7a3 | COX7S_HUMAN | Putative cytochrome c oxidase subunit 7A3, mitochondrial | Pfam PF02238 |
| 16 | Cox7b | COX7B_HUMAN | Cytochrome c oxidase subunit 7B, mitochondrial | Pfam PF05392 |
| 17 | Cox7c | COX7C_HUMAN | Cytochrome c oxidase subunit 7C, mitochondrial | Pfam PF02935 |
| 18 | Cox7r | COX7R_HUMAN | Cytochrome c oxidase subunit 7A-related protein, mitochondrial | Pfam PF02238 |
| 19 | Cox8a | COX8A_HUMAN | Cytochrome c oxidase subunit 8A, mitochondrial P | Pfam PF02285 |
| 20 | Cox8c | COX8C_HUMAN | Cytochrome c oxidase subunit 8C, mitochondrial | Pfam PF02285 |
| Assembly subunits[10][11][12] | ||||
| 1 | Coa1 | COA1_HUMAN | Cytochrome c oxidase assembly factor 1 homolog | Pfam PF08695 |
| 2 | Coa3 | COA3_HUMAN | Cytochrome c oxidase assembly factor 3 homolog, mitochondrial | Pfam PF09813 |
| 3 | Coa4 | COA4_HUMAN | Cytochrome c oxidase assembly factor 4 homolog, mitochondrial | Pfam PF06747 |
| 4 | Coa5 | COA5_HUMAN | Cytochrome c oxidase assembly factor 5 | Pfam PF10203 |
| 5 | Coa6 | COA6_HUMAN | Cytochrome c oxidase assembly factor 6 homolog | Pfam PF02297 |
| 6 | Coa7 | COA7_HUMAN | Cytochrome c oxidase assembly factor 7, | Pfam PF08238 |
| 7 | Cox11 | COX11_HUMAN | Cytochrome c oxidase assembly protein COX11 mitochondrial | Pfam PF04442 |
| 8 | Cox14 | COX14_HUMAN | Cytochrome c oxidase assembly protein | Pfam PF14880 |
| 9 | Cox15 | COX15_HUMAN | Cytochrome c oxidase assembly protein COX15 homolog | Pfam PF02628 |
| 10 | Cox16 | COX16_HUMAN | Cytochrome c oxidase assembly protein COX16 homolog mitochondrial | Pfam PF14138 |
| 11 | Cox17 | COX17_HUMAN | Cytochrome c oxidase copper chaperone | Pfam PF05051 |
| 12 | Cox18[13] | COX18_HUMAN | Mitochondrial inner membrane protein (Cytochrome c oxidase assembly protein 18) | Pfam PF02096 |
| 13 | Cox19 | COX19_HUMAN | Cytochrome c oxidase assembly protein | Pfam PF06747 |
| 14 | Cox20 | COX20_HUMAN | Cytochrome c oxidase protein 20 homolog | Pfam PF12597 |
Biochemistry
Summary reaction:
- 4 Fe2+-cytochrome c + 8 H+in + O2 → 4 Fe3+-cytochrome c + 2 H2O + 4 H+out
Two electrons are passed from two cytochrome c's, through the CuA and cytochrome a sites to the cytochrome a3- CuB binuclear center, reducing the metals to the Fe2+ form and Cu+. The hydroxide ligand is protonated and lost as water, creating a void between the metals that is filled by O2. The oxygen is rapidly reduced, with two electrons coming from the Fe2+cytochrome a3, which is converted to the ferryl oxo form (Fe4+=O). The oxygen atom close to CuB picks up one electron from Cu+, and a second electron and a proton from the hydroxyl of Tyr(244), which becomes a tyrosyl radical: The second oxygen is converted to a hydroxide ion by picking up two electrons and a proton. A third electron arising from another cytochrome c is passed through the first two electron carriers to the cytochrome a3- CuB binuclear center, and this electron and two protons convert the tyrosyl radical back to Tyr, and the hydroxide bound to CuB2+ to a water molecule. The fourth electron from another cytochrome c flows through CuA and cytochrome a to the cytochrome a3- CuB binuclear center, reducing the Fe4+=O to Fe3+, with the oxygen atom picking up a proton simultaneously, regenerating this oxygen as a hydroxide ion coordinated in the middle of the cytochrome a3- CuB center as it was at the start of this cycle. The net process is that four reduced cytochrome c's are used, along with 4 protons, to reduce O2 to two water molecules.
Inhibition
Cyanide, sulfide, azide, and carbon monoxide[14] all bind to cytochrome c oxidase, thus competitively inhibiting the protein from functioning, which results in chemical asphyxiation of cells. Methanol in methylated spirits is converted into formic acid, which also inhibits the same oxidase system. High levels of ATP can allosterically inhibit cytochrome c oxidase, binding from within the mitochondrial matrix.[15]
Subcellular distribution
Cytochrome c oxidase has 3 subunits which are encoded by mitochondrial DNA. Of these 3 subunits encoded by mitochondrial DNA, two have been identified in extramitochondrial locations. In pancreatic acinar tissue, these subunits were found in zymogen granules. Additionally, in the anterior pituitary, relatively high amounts of these subunits were found in growth hormone secretory granules.[16] The extramitochondrial function of these cytochrome c oxidase subunits has not yet been characterized. Besides cytochrome c oxidase subunits, extramitochondrial localization has also been observed for large numbers of other mitochondrial proteins.[17][18] This raises the possibility about existence of yet unidentified specific mechanisms for protein translocation from mitochondria to other cellular destinations.[16][18][19]
Clinical significance
Defects involving genetic mutations altering cytochrome c oxidase (COX) functionality or structure can result in severe, often fatal metabolic disorders. Such disorders usually manifest in early childhood and affect predominantly tissues with high energy demands (brain, heart, muscle). Among the many classified mitochondrial diseases, those involving dysfunctional COX assembly are thought to be the most severe.[20]
The vast majority of COX disorders are linked to mutations in nuclear-encoded proteins referred to as assembly factors, or assembly proteins. These assembly factors contribute to COX structure and functionality, and are involved in several essential processes, including transcription and translation of mitochondrion-encoded subunits, processing of preproteins and membrane insertion, and cofactor biosynthesis and incorporation.[21]
Currently, mutations have been identified in seven COX assembly factors: SURF1, SCO1, SCO2, COX10, COX15, COX20, COA5 and LRPPRC. Mutations in these proteins can result in altered functionality of sub-complex assembly, copper transport, or translational regulation. Each gene mutation is associated with the etiology of a specific disease, with some having implications in multiple disorders. Disorders involving dysfunctional COX assembly via gene mutations include Leigh syndrome, cardiomyopathy, leukodystrophy, anemia, and sensorineural deafness.
Histochemistry
COX histochemistry is used for mapping regional brain metabolism in animals, since there is a direct relation between enzyme activity and neuronal activity.[22] Such brain mapping has been accomplished in spontaneous mutant mice with cerebellar disease such as reeler[23] and a transgenic model of Alzheimer's disease.[24] This technique has also been used to map learning activity in animal brain.[25]
Additional images
-
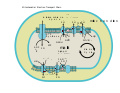
ETC
-
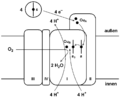
Complex IV
See also
- Cytochrome c oxidase subunit I
- Cytochrome c oxidase subunit II
- Cytochrome c oxidase subunit III
- Heme a
References
- ↑ Yoshikawa, Shinya; Shimada, Atsuhiro; Shinzawa-Itoh, Kyoko (2015). "Chapter 4 Respiratory Conservation of Energy with Dioxygen: Cytochrome c Oxidase". In Peter M.H. Kroneck and Martha E. Sosa Torres. Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences 15. Springer. pp. 89–130. doi:10.1007/978-3-319-12415-5_4.
- ↑ Yee, Gereon M.; Tolman, William B. (2015). "Chapter 5, Section 2.3 Iron-Porphyrin/Copper Complexes as Cytochrome c Oxidase Models". In Peter M.H. Kroneck and Martha E. Sosa Torres. Sustaining Life on Planet Earth: Metalloenzymes Mastering Dioxygen and Other Chewy Gases. Metal Ions in Life Sciences 15. Springer. pp. 153–157. doi:10.1007/978-3-319-12415-5_5.
- ↑ Balsa E, Marco R, Perales-Clemente E, Szklarczyk R, Calvo E, Landázuri MO, Enríquez JA (September 2012). "NDUFA4 is a subunit of complex IV of the mammalian electron transport chain". Cell Metab. 16 (3): 378–86. doi:10.1016/j.cmet.2012.07.015. PMID 22902835.
- ↑ Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S (August 1995). "Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A". Science 269 (5227): 1069–74. doi:10.1126/science.7652554. PMID 7652554.
- ↑ Voet, Donald (2010). Biochemistry. New York: J. Wiley & Sons. pp. 865–866. ISBN 0-470-57095-4.
- ↑ Khalimonchuk O, Rödel G (December 2005). "Biogenesis of cytochrome c oxidase". Mitochondrion 5 (6): 363–88. doi:10.1016/j.mito.2005.08.002. PMID 16199211.
- ↑ Fontanesi F, Soto IC, Horn D, Barrientos A (December 2006). "Assembly of mitochondrial cytochrome c-oxidase, a complicated and highly regulated cellular process". Am. J. Physiol., Cell Physiol. 291 (6): C1129–47. doi:10.1152/ajpcell.00233.2006. PMID 16760263.
- ↑ Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH (1998). "Electron transfer by domain movement in cytochrome bc1.". Nature 392 (6677): 677–84. doi:10.1038/33612. PMID 9565029.
- ↑ Kaila VR, Oksanen E, Goldman A, Bloch DA, Verkhovsky MI, Sundholm D, Wikström M (2011). "A combined quantum chemical and crystallographic study on the oxidized binuclear center of cytochrome c oxidase.". Biochim Biophys Acta 1807 (7): 769–78. doi:10.1016/j.bbabio.2010.12.016. PMID 21211513.
- ↑ Szklarczyk R, Wanschers BF, Cuypers TD, Esseling JJ, Riemersma M, van den Brand MA, Gloerich J, Lasonder E, van den Heuvel LP, Nijtmans LG, Huynen MA (2012). "Iterative orthology prediction uncovers new mitochondrial proteins and identifies C12orf62 as the human ortholog of COX14, a protein involved in the assembly of cytochrome c oxidase.". Genome Biol 13 (2): R12. doi:10.1186/gb-2012-13-2-r12. PMC 3334569. PMID 22356826.
- ↑ Mick DU, Dennerlein S, Wiese H, Reinhold R, Pacheu-Grau D, Lorenzi I, Sasarman F, Weraarpachai W, Shoubridge EA, Warscheid B, Rehling P (2012). "MITRAC links mitochondrial protein translocation to respiratory-chain assembly and translational regulation.". Cell 151 (7): 1528–41. doi:10.1016/j.cell.2012.11.053. PMID 23260140.
- ↑ Kozjak-Pavlovic V, Prell F, Thiede B, Götz M, Wosiek D, Ott C, Rudel T (2014). "C1orf163/RESA1 is a novel mitochondrial intermembrane space protein connected to respiratory chain assembly.". J Mol Biol 426 (4): 908–20. doi:10.1016/j.jmb.2013.12.001. PMID 24333015.
- ↑ Gaisne M, Bonnefoy N (2006). "The COX18 gene, involved in mitochondrial biogenesis, is functionally conserved and tightly regulated in humans and fission yeast.". FEMS Yeast Res 6 (6): 869–82. doi:10.1111/j.1567-1364.2006.00083.x. PMID 16911509.
- ↑ Alonso JR, Cardellach F, López S, Casademont J, Miró O (September 2003). "Carbon monoxide specifically inhibits cytochrome c oxidase of human mitochondrial respiratory chain". Pharmacol. Toxicol. 93 (3): 142–6. doi:10.1034/j.1600-0773.2003.930306.x. PMID 12969439.
- ↑ Arnold S, Kadenbach B (October 1997). "Cell respiration s controlled by ATP, an allosteric inhibitor of cytochrome-c oxidase.". Eur J Biochem 249: 350–254. doi:10.1111/j.1432-1033.1997.t01-1-00350.x. PMID 9363790.
- 1 2 Sadacharan SK, Singh B, Bowes T, Gupta RS (2005). "Localization of mitochondrial DNA encoded cytochrome c oxidase subunits I and II in rat pancreatic zymogen granules and pituitary growth hormone granules". Histochem. Cell Biol. 124 (5): 409–21. doi:10.1007/s00418-005-0056-2. PMID 16133117.
- ↑ Gupta RS, Ramachandra NB, Bowes T, Singh B (2008). "Unusual cellular disposition of the mitochondrial molecular chaperones Hsp60, Hsp70 and Hsp10". Novartis Found. Symp. 291: 59–68; discussion 69–73, 137–40. doi:10.1002/9780470754030.ch5. PMID 18575266.
- 1 2 Soltys BJ, Gupta RS (2000). "Mitochondrial proteins at unexpected cellular locations: export of proteins from mitochondria from an evolutionary perspective". Int. Rev. Cytol. 194: 133–96. doi:10.1016/s0074-7696(08)62396-7. PMID 10494626.
- ↑ Soltys BJ, Gupta RS (1999). "Mitochondrial-matrix proteins at unexpected locations: are they exported?". Trends Biochem. Sci. 24 (5): 174–7. doi:10.1016/s0968-0004(99)01390-0. PMID 10322429.
- ↑ Pecina P, Houstková H, Hansíková H, Zeman J, Houstek J (2004). "Genetic defects of cytochrome c oxidase assembly" (PDF). Physiol Res. 53 Suppl 1: S213–23. PMID 15119951.
- ↑ Zee JM, Glerum DM (December 2006). "Defects in cytochrome oxidase assembly in humans: lessons from yeast". Biochem. Cell Biol. 84 (6): 859–69. doi:10.1139/o06-201. PMID 17215873.
- ↑ Wong-Riley MT (1989). "Cytochrome oxidase: an endogenous metabolic marker for neuronal activity.". Trends Neurosci. 12 (3): 94–111. doi:10.1016/0166-2236(89)90165-3. PMID 2469224.
- ↑ Strazielle C, Hayzoun K, Derer M, Mariani J, Lalonde R (April 2006). "Regional brain variations of cytochrome oxidase activity in Relnrl-orl mutant mice.". J. Neurosci. Res. 83 (5): 821–31. doi:10.1002/jnr.20772. PMID 16511878.
- ↑ Strazielle C, Sturchler-Pierrat C, Staufenbiel M, Lalonde R (2003). "Regional brain cytochrome oxidase activity in beta-amyloid precursor protein transgenic mice with the Swedish mutation.". Neuroscience 118 (4): 1151–63. doi:10.1016/S0306-4522(03)00037-X. PMID 12732258.
- ↑ Conejo NM, González-Pardo H, Gonzalez-Lima F, Arias JL (2010). "Spatial learning of the water maze: progression of brain circuits mapped with cytochrome oxidase histochemistry.". Neurobiol. Learn. Mem. 93 (3): 362–71. doi:10.1016/j.nlm.2009.12.002. PMID 19969098.
External links
- The Cytochrome Oxidase home page at Rice University
- Interactive Molecular model of cytochrome c oxidase (Requires MDL Chime)
- UMich Orientation of Proteins in Membranes families/superfamily-4
- Cytochrome-c Oxidase at the US National Library of Medicine Medical Subject Headings (MeSH)
| ||||||||||||||||||||||||||||||||||
| ||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||
| ||||||||||||||||||
|
