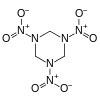
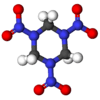
RDX
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
1,3,5-Trinitroperhydro-1,3,5-triazine | |||
| Other names
RDX cyclonite, hexogen 1,3,5-Trinitro-1,3,5-triazacyclohexane 1,3,5-Trinitrohexahydro-s-triazine Cyclotrimethylenetrinitramine Hexahydro-1,3,5-trinitro-s-triazine Trimethylenetrinitramine | |||
| Identifiers | |||
| 121-82-4 | |||
| ChEBI | CHEBI:24556 | ||
| ChemSpider | 8177 | ||
| Jmol interactive 3D | Image | ||
| PubChem | 8490 | ||
| UN number | 0072, 0391, 0483 | ||
| |||
| |||
| Properties | |||
| C3H6N6O6 | |||
| Molar mass | 222.12 g·mol−1 | ||
| Appearance | Colorless crystals | ||
| Density | 1.82 g/cm3 | ||
| Melting point | 205.5 °C (401.9 °F; 478.6 K) | ||
| Boiling point | 234 °C (453 °F; 507 K) | ||
| insoluble[1] | |||
| Explosive data | |||
| Shock sensitivity | Low | ||
| Friction sensitivity | Low | ||
| Detonation velocity | 8750 m/s | ||
| RE factor | 1.60 | ||
| Hazards | |||
| Main hazards | Explosive, detonates on contact with mercury fulminate[1] | ||
| Flash point | Explosive[1] | ||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
none[1] | ||
| REL (Recommended) |
TWA 1.5 mg/m3 ST 3 mg/m3 [skin][1] | ||
| IDLH (Immediate danger |
N.D.[1] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
RDX, an initialism for Research Department explosive,[2] is an explosive nitroamine widely used in military and industrial applications. It was developed as an explosive which was more powerful than TNT, and it saw wide use in World War II. RDX is also known as Research Department Formula X,[3] cyclonite, hexogen (particularly in German and German-influenced languages), and T4. Its chemical name is cyclotrimethylenetrinitramine; name variants include cyclotrimethylene-trinitramine and cyclotrimethylene trinitramine.
In its pure, synthesized state RDX is a white, crystalline solid. It is often used in mixtures with other explosives and plasticizers, phlegmatizers or desensitizers. RDX is stable in storage and is considered one of the most powerful and brisant of the military high explosives.[4]
Name
RDX is also known, but less commonly, as cyclonite, hexogen (particularly in Russian, German and German-influenced languages), T4 and chemically as cyclotrimethylenetrinitramine. Tenney L Davis, writing in the US in 1943, stated it was generally known in the US as cyclonite; the Germans called it Hexogen, the Italians T4.[5] In the 1930s, the Royal Arsenal, Woolwich, started investigating cyclonite as an explosive to use against German U-boats that were being built with thicker hulls. Britain wanted an explosive that was more powerful than TNT. For security reasons, Britain termed Cyclonite as "Research Department Explosive" (R.D.X.).[2] The term RDX appeared in the United States in 1946, but the name RDX is given without explanation.[6] The first public reference in the United Kingdom to the name RDX, or R.D.X. to use the official title, appeared in 1948; its authors were the Managing Chemist, ROF Bridgwater, the Chemical Research and Development Department, Woolwich, and the Director of Royal Ordnance Factories, Explosives; again, it was referred to as simply RDX.[7]
Usage

RDX was widely used during World War II, often in explosive mixtures with TNT such as Torpex, Composition B, Cyclotols, and H6. RDX was used in one of the first plastic explosives. RDX is believed to have been used in many bomb plots including terrorist plots. The bouncing bomb depth charges used in the "Dambusters Raid" each contained 6,600 pounds (3,000 kg) of Torpex.[8]
RDX forms the base for a number of common military explosives:
- Composition A: Granular explosive consisting of RDX and plasticizing wax. Such as, composition A-5 (RDX coated with 1.5% stearic acid) and composition A-3 (91% RDX coated with 9% wax)
- Composition B: Castable mixtures of 60% RDX and 40% TNT, with an extra 1% of wax added (desensitizer)
- Composition C: The original composition C was used in World War II, but there have been subsequent variations including C-2, C-3, and C-4. C-4 consists of RDX (91%), a plasticizer (which can be dioctyl adipate {DOA}, diethylhexyl, or dioctyl sebacate) (5.3%), a binder, which is usually polyisobutylene (2.1%), SAE 10 non-detergent motor oil (1.6%).
- Composition CH-6: 97.5% RDX, 1.5% calcium stearate, 0.5% polyisobutylene, and 0.5% graphite.[9]
- DBX (Depth Bomb Explosive): Castable mixture consisting of 21% RDX, 21% ammonium nitrate, 40% TNT, and 18% powdered aluminium. Developed during World War II, it was to be used in underwater munitions as a substitute for Torpex employing only half the amount of then-strategic RDX.[4][10] As the supply of RDX became more adequate, the mixture was shelved.
- Cyclotol: Castable mixture of RDX (50–80%) with TNT (20–50%) designated by the amount of RDX/TNT, such as Cyclotol 70/30.
- HBX: Castable mixtures of RDX, TNT, powdered aluminium, and D-2 wax with calcium chloride.
- H-6: Castable mixture of RDX, TNT, powdered aluminum, and paraffin wax (used as a phlegmatizing agent).
- PBX: RDX is also used as a major component of many polymer-bonded explosives (PBX). RDX-based PBXs typically consist of RDX and a polymer/co-polymer binder. Examples of RDX-based PBX formulations include, but are not limited to: PBX-9007, PBX-9010, PBX-9205, PBX-9407, PBX-9604, PBXN-106, PBXN-3, PBXN-6, PBXN-10, PBXN-201, PBX-0280, PBX Type I, PBXC-116, PBXAF-108, etc.
- Semtex (trade name): Plastic demolition explosives containing RDX and PETN as major energetic components.
- Torpex: 42% RDX, 40% TNT, and 18% powdered aluminium. The mixture was designed during World War II and used mainly in underwater ordnance.
Outside military applications, RDX is also used in controlled demolition to raze structures. The demolition of the Jamestown Bridge in the US state of Rhode Island is one instance where RDX shaped charges were used to remove the span.
Properties
The velocity of detonation of RDX at a density of 1.76 g/cm3 is 8750 m/s.
It is a colourless solid, of crystal density 1.82 g/cm3. It is obtained by reacting white fuming nitric acid (WFNA) with hexamine, producing dinitromethane and ammonium nitrate as byproducts.[11]
- Hexamine + 10 nitric acid → RDX + methylene dinitrate + ammonium nitrate + 3 water
- (CH2)6N4 + 10 HNO3 → (CH2-N-NO2)3 + 3 CH2(NO3)2 + NH4NO3 + 3 H2O
It is a heterocycle and has the molecular shape of a ring. It starts to decompose at about 170 °C and melts at 204 °C. Its structural formula is: hexahydro-1,3,5-trinitro-1,3,5-triazine or (CH2-N-NO2)3.
At room temperature, it is very stable. It burns rather than explodes and detonates only with a detonator, being unaffected even by small arms fire. (This is one of the properties that make it a useful military explosive.) It is less sensitive than pentaerythritol tetranitrate (PETN). Under normal conditions, RDX has a figure of insensitivity of exactly 80 (RDX defines the reference point.).
RDX sublimates in vacuum, which limits its use in pyrotechnic fasteners for spacecraft.
RDX when exploded in air has about 1.5 times the explosive power of TNT per unit weight and about 2.0 times per unit volume.[12][13]
History
RDX was used by both sides in World War II. The US produced about 15,000 long tons (15,000 t) per month during WWII and Germany about 7,000 long tons (7,100 t) per month.[14] RDX had the major advantages of possessing greater explosive power than TNT used in the First World War, and requiring no additional raw materials for its manufacture.[14]
Germany
The discovery of RDX dates from 1898 when Georg Friedrich Henning obtained a German patent (patent No. 104280) for its manufacture, by nitrating hexamine nitrate (hexamethylenetetramine nitrate) with concentrated nitric acid.[15][16] In this 1898 patent, its properties as a medical compound were mentioned; however, three further German patents obtained by Henning in 1916 proposed its use in smokeless propellants.[15] The German military started investigating its use in 1920 and referred to it as hexogen.[17] Research and development findings were not published further until Edmund von Herz,[18] described as an Austrian and later a German citizen, obtained a British patent in 1921[19] and a United States patent in 1922.[20] Both patent claims were initiated in Austria; and described the manufacture of RDX by nitrating hexamethylenetetramine.[19][20] The British patent claims included the manufacture of RDX by nitration, its use with or without other explosives, and its use as a bursting charge and as an initiator.[19] The US patent claim was for the use of a hollow explosive device containing RDX and a detonator cap containing RDX.[20] In the 1930s, Germany developed improved production methods.[17]
During the Second World War, Germany used the code names W Salt, SH Salt, K-method, the E-method and the KA-method. These represented the names of the developers of the various chemical processes used to prepare RDX. The W-method was developed by Wolfram in 1934 and gave RDX the code name "W-Salz". It used sulfamic acid, formaldehyde and nitric acid.[21] SH-Salz (SH salt) was from Schnurr who developed a batch-process in 1937–38 based on nitrating hexamine.[22] The K-method was from Knöffler and was based on adding ammonium nitrate to the hexamine / nitric acid process.[23] The E-method was developed by Ebele, in Germany, and turned out to be identical to the Ross and Schiessler process described later.[24] The KA-method was developed by Knöffler, in Germany, and turned out to be identical to the Bachmann process described later.[25]
The explosive shells fired by the MK 108 cannon and the warhead of the R4M rocket, both used in Luftwaffe fighter aircraft as offensive armament, both used hexogen as their explosive base.
UK
In the United Kingdom (UK), RDX was manufactured from 1933 by the Research Department in a pilot plant at the Royal Arsenal in Woolwich, London, a larger pilot plant being built at the RGPF Waltham Abbey just outside London in 1939.[26][27] In 1939 a twin-unit industrial-scale plant was designed to be installed at a new 700 acres (280 ha) site, ROF Bridgwater, away from London; production of RDX started at Bridgwater on one unit in August 1941.[26][28] The ROF Bridgwater plant brought in ammonia and methanol as raw materials: the methanol was converted to formaldehyde and some of the ammonia converted to nitric acid, which was concentrated for RDX production.[7] The rest of the ammonia was reacted with formaldehyde to produce hexamine. The hexamine plant was supplied by Imperial Chemical Industries; it incorporated some features based on data obtained from the United States (US).[7] RDX was produced by continually adding hexamine and concentrated nitric acid to a cooled mixture of hexamine and nitric acid in the nitrator.[7] The RDX was purified and processed for its intended use; recovery and reuse of some methanol and nitric acid was also carried out.[7] The hexamine-nitration and RDX purification plants were duplicated (i.e. twin-unit) to provide some insurance against loss of production due to fire, explosion or air attack.[26]
The United Kingdom and British Empire were fighting without allies against Nazi Germany until the middle of 1941 and had to be self-sufficient. At that time (1941), the UK had the capacity to produce 70 long tons (71 t) (160,000 lb) of RDX per week; both Canada, an allied country and self-governing dominion within the British Empire, and the US were looked upon to supply ammunition and explosives, including RDX.[29] By 1942 the Royal Air Force's annual requirement was forecast to be 52,000 long tons (53,000 t) of RDX, much of which came from North America (Canada and the US).[28]
Canada
A different method of production to the Woolwich process was found and used in Canada, possibly at the McGill University Department of Chemistry. This was based on reacting paraformaldehyde and ammonium nitrate in acetic anhydride.[30] A UK patent application was made by Robert Walter Schiessler (Pennsylvania State College) and James Hamilton Ross (McGill, Canada) in May 1942; the UK patent was issued in December 1947.[31] Gilman states that the same method of production had been independently discovered by Ebele in Germany prior to Schiessler and Ross, but that this was not known by the Allies.[15][30] Urbański provides details of five methods of production, and he refers to this method as the (German) E-method.[24]
UK, US and Canadian production and development
At the beginning of the 1940s, the major US explosive manufacturers, E. I. du Pont de Nemours & Company and Hercules, had several decades of experience of manufacturing trinitrotoluene (TNT) and had no wish to experiment with new explosives. US Army Ordnance held the same viewpoint and wanted to continue using TNT.[32] RDX had been tested by Picatinny Arsenal in 1929 and it was regarded as too expensive and too sensitive.[29] The Navy proposed to continue using ammonium picrate.[32] In contrast, the National Defense Research Committee (NDRC), who had visited The Royal Arsenal, Woolwich, did not share the view that new explosives were unnecessary.[32] James B. Conant, chairman of Division B, wished to involve academic research into this area. Conant therefore set up an Experimental Explosives Research Laboratory at the Bureau of Mines, Bruceton, Pennsylvania, using Office of Scientific Research and Development (OSRD) funding.[29]
Woolwich method
In 1941, the UK's Tizard Mission visited the US Army and Navy departments and part of the information handed over included details of the "Woolwich" method of manufacture of RDX and its stabilisation by mixing it with beeswax.[29] The UK was asking that the US and Canada, combined, supply 220 short tons (200 t) (440,000 lb) of RDX per day.[29] A decision was taken by William H. P. Blandy, Chief of the Bureau of Ordnance to adopt RDX for use in mines and torpedoes.[29] Given the immediate need for RDX, the US Army Ordnance, at Blandy's request, built a plant that just copied the equipment and process used at Woolwich. The result was the Wabash River Ordnance Works run by E. I. du Pont de Nemours & Company.[33] At that time, this works had the largest nitric acid plant in the world.[29] The Woolwich process was expensive; it needed 11 pounds (5.0 kg) of strong nitric acid for every pound of RDX.[34]
By early 1941, the NDRC was researching new processes.[34] The Woolwich or direct nitration process has at least two serious disadvantages: (1) it used large amounts of nitric acid and (2) at least one-half of the formaldehyde is lost. One mole of hexamethylenetetramine could produce at most one mole of RDX.[35] At least three laboratories with no previous explosive experience were tasked to develop better production methods for RDX; they were based at Cornell, Michigan, and Penn State universities.[29][36] Werner Emmanuel Bachmann, from Michigan, successfully developed the "combination process" by combining the Canadian process with direct nitration.[25][29] The combination process required large quantities of acetic anhydride instead of nitric acid in the old British "Woolwich process". Ideally, the combination process could produce two moles of RDX from each mole of hexamethylenetetramine.[35]
The vast production of RDX could not continue to rely on the use of natural beeswax to desensitize the RDX. A substitute stabilizer based on petroleum was developed at the Bruceton Explosives Research Laboratory.[29]
Bachmann process
The NDRC tasked three companies to develop pilot plants. They were the Western Cartridge Company, E. I. du Pont de Nemours & Company and Tennessee Eastman Company, part of Eastman Kodak.[29] At the Eastman Chemical Company (TEC), a leading manufacturer of acetic anhydride, Werner Emmanuel Bachmann successfully developed a continuous-flow manufacturing process for RDX. RDX was crucial to the war effort and the current batch-production process could not keep up. In February 1942, TEC built the Wexler Bend pilot plant and began producing small amounts of RDX. This led to the US government authorizing TEC to design and build Holston Ordnance Works (H.O.W.) in June 1942. By April 1943, RDX was being manufactured there.[37] At the end of 1944, the Holston plant and the Wabash River Ordnance Works (which used the Woolwich process) were making 25,000 short tons (23,000 t) (50 million pounds) of Composition B per month.[38]
The US Bachmann process for RDX was found to be richer in HMX than the United Kingdom's RDX. This later led to a RDX plant using the Bachmann process being set up at ROF Bridgwater in 1955, to produce both RDX and HMX.
Military compositions
The United Kingdom's intention in World War II was to use "desensitised" RDX. In the original Woolwich process, RDX was phlegmatized with beeswax, but later paraffin wax was used, based on the work carried out at Bruceton. In the event the UK was unable to obtain sufficient RDX to meet its needs, some of the shortfall was met by substituting amatol, a mixture of ammonium nitrate and TNT.[28]
Karl Dönitz was reputed to have claimed that "an aircraft can no more kill a U-boat than a crow can kill a mole".[39] However, by May 1942 Wellington bombers began to deploy depth charges containing Torpex, a mixture of RDX, TNT and aluminium, which had up to 50 percent more destructive power than TNT-filled depth charges.[39] Considerable quantities of the RDX–TNT mixture were produced at the Holston Ordnance Works, with Tennessee Eastman developing an automated mixing and cooling process based around the use of stainless steel conveyor belts.[13]
Terrorism
The 1993 Bombay bombings used RDX placed into several vehicles as bombs. RDX was the main component used for the 2006 Mumbai train bombings and the Jaipur bombings in 2008.[40][41] It is also believed to be the explosive used in the 1999 Russian apartment bombings,[42] 2004 Russian aircraft bombings,[43] and 2010 Moscow Metro bombings.[44]
Ahmed Ressam, the al-Qaeda Millennium Bomber, used a small quantity of RDX as one of the components in the explosives that he prepared to bomb Los Angeles International Airport on New Year's Eve 1999/2000; the combined explosives could have produced a blast forty times greater than that of a devastating car bomb.[45][46]
In July 2012, the Kenyan government arrested two Iranian nationals and charged them with illegal possession of 15 kilograms (33 pounds) of RDX. According to the Kenyan Police, the Iranians planned to use the RDX for "attacks on Israel, US, UK and Saudi Arabian targets".[47]
RDX was used to assassinate Lebanese prime minister Rafiq Hariri on February 14, 2005.[48]
Toxicity
RDX has caused convulsions (seizures) in military field personnel ingesting it, and in munition workers inhaling its dust during manufacture. The substance's toxicity has been studied for many years.[49] At least one fatality was attributed to RDX toxicity in a European munitions manufacturing plant.[50] The substance has low to moderate toxicity with a possible human carcinogen classification.[51] However, further research is ongoing and this classification may be revised by the United States Environmental Protection Agency (EPA).[52][53] Remediating RDX contaminated water supplies has proven to be successful.[54]
Biodegradation
RDX produces high amounts of carbon dioxide and nitrous oxide when treated with sewage sludge or the fungus Phanaerocheate chrysosporium. A change of a N-NO2 or C-H bond of cyclic nitramine leads to cleavage of the ring, when the C-N bonds weaken.[55] Both wild and transgenic plants can phytoremediate explosives from soil and water.[56]
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0169". National Institute for Occupational Safety and Health (NIOSH).
- 1 2 MacDonald and Mack Partnership (1984, p. 18)
- ↑ The National Archives, London, DEFE 15/2406 dated September 1942, refers to this material as Research Department Formula X. Their original file reference was Explosives Report 275/2. The Research Department at Woolwich Arsenal named it thus as they invented the substance.
- 1 2 Department of the Army Technical Manual TM 9-1300-214: Military Explosives. Headquarters, Department of the Army (United States).
- ↑ Davis, Tenney L. (1943), The Chemistry of Powder and Explosives II, New York: John Wiley & Sons Inc., p. 396
- ↑ Baxter III 1968, pp. 27, 42, 255–259
- 1 2 3 4 5 Simmons, W.H.; Forster, A.; Bowden, R. C. (August 1948), "The Manufacture of R.D.X. in Great Britain: Part II – Raw Materials and Ancillary Processes", The Industrial Chemist 24: 530–545; Simmons, W.H.; Forster, A.; Bowden, R. C. (September 1948), "The Manufacture of R.D.X. in Great Britain: Part III – Production of the Explosive", The Industrial Chemist 24: 593–601
- ↑ Sweetman, John (2002) The Dambusters Raid. London: Cassell Military Paperbacks. p. 144.
- ↑ Hampton, L. D. (June 15, 1960), The Development of RDX Composition CH-6 (PDF), White Oak, MD: U. S. Naval Ordnance Laboratory, NavOrd Report 680
- ↑ US Explosive Ordnance; Ordnance Pamphlet OP 1664 1. Washington, D.C.: Navy Department, Bureau of Ordnance. May 28, 1947. pp. 3–4. OP 1664 states 21% "aluminum nitrate", but the immediately following text refers to ammonium nitrate.
- ↑ Luo, K.-M.; Lin, S.-H.; Chang, J.-G.; Huang, T.-H. (2002), "Evaluations of kinetic parameters and critical runaway conditions in the reaction system of hexamine-nitric acid to produce RDX in a non-isothermal batch reactor", Journal of Loss Prevention in the Process Industries 15 (2): 119–127, doi:10.1016/S0950-4230(01)00027-4.
- ↑ Elderfield (1960, p. 8)
- 1 2 Baxter III (1968, pp. 257 & 259)
- 1 2 Urbański (1967, p. 78)
- 1 2 3 Urbański (1967, pp. 77–119)
- ↑ DE 104280, Henning, Georg Friedrich, issued June 14, 1899
- 1 2 Hexogen. economypoint.org, citing Gartz, Jochen (2007), Vom griechischen Feuer zum Dynamit: eine Kulturgeschichte der Explosivstoffe [From Greek fire to dynamite: A cultural history of explosives] (in German), Hamburg: E. S. Mittler & Sohn, ISBN 978-3-8132-0867-2
- ↑ Urbański (1967, p. 125) credits "G. C. V. Herz" for the patent, but the patentee is Edmund von Herz.
- 1 2 3 GB 145791, von Herz, Edmund, "Improvements relating to Explosives", issued March 17, 1921
- 1 2 3 US 1402693, von Herz, Edmund, "Explosive", issued January 3, 1922
- ↑ Urbański (1967, pp. 107–109)
- ↑ Urbański (1967, pp. 104–105)
- ↑ Urbański (1967, pp. 105–107)
- 1 2 Urbański (1967, pp. 109–110)
- 1 2 Urbański (1967, pp. 111–113)
- 1 2 3 Cocroft, Wayne D. (2000), Dangerous Energy: The archaeology of gunpowder and military explosives manufacture, Swindon: English Heritage, pp. 210–211, ISBN 1-85074-718-0
- ↑ Akhavan, Jacqueline (2004), The Chemistry of Explosives, Cambridge, UK: Royal Society of Chemistry, ISBN 0-85404-640-2
- 1 2 3 Hornby, William (1958), Factories and Plant, History of the Second World War: United Kingdom Civil Series, London: Her Majesty's Stationery Office; Longmans, Green and Co., pp. 112–114
- 1 2 3 4 5 6 7 8 9 10 11 Baxter III (1968, pp. 253–239)
- 1 2 Gilman, Henry (1953), "The Chemistry of Explosives", Organic Chemistry an Advanced Treatise III, Wiley; Chapman & Hall, p. 985
- ↑ GB 595354, Schiessler, Robert Walter & James Hamilton Ross, "Method of Preparing 1.3.5. Trinitro Hexahydro S-Triazine", issued December 3, 1947
- 1 2 3 Baxter III (1968, pp. 253–254)
- ↑ MacDonald and Mack Partnership (1984, p. 19)
- 1 2 MacDonald and Mack Partnership (1984, p. 13) These pages need to be checked. Page 13 may actually be page 18.
- 1 2 Elderfield (1960, p. 6)
- ↑ These were not the only laboratories to work on RDX, Gilman's 1953 account of the Ross-Schiessler method was based on unpublished work from laboratories at the Universities of Michigan, Pennsylvania, Cornell, Harvard, Vanderbilt, McGill (Canada), Bristol (UK), Sheffield (UK), Pennsylvania State College and the UK's Research Department.
- ↑ Bachmann, W. E.; Sheehan, John C. (1949), "A New Method of Preparing the High Explosive RDX", Journal of the American Chemical Society 71 (5): 1842–1845, doi:10.1021/ja01173a092
- ↑ MacDonald and Mack Partnership (1984, p. 32)
- 1 2 Baxter III (1968), p. 42
- ↑ Singh, Anil (October 2, 2006). "Mumbai". The Times of India.
- ↑ "Jaipur blasts: RDX used, HuJI suspected". Times of India. May 14, 2008. Retrieved May 13, 2011.
- ↑ "Debate on Cause of Moscow Blast Heats Up". New York Times. September 10, 1999. Retrieved November 14, 2011.
- ↑ "Explosive Suggests Terrorists Downed Plane, Russia Says". New York Times. August 28, 2004. Retrieved November 14, 2011.
- ↑ "Moscow Metro bombing masterminds 'will be destroyed'". BBC News. March 29, 2010. Retrieved April 2, 2010.
- ↑ U.S. Court of Appeals for the Ninth Circuit (February 2, 2010). "U.S. v. Ressam" (PDF). Retrieved February 27, 2010.
- ↑ "Complaint; U.S. v. Ressam" (PDF). NEFA Foundation. December 1999. Retrieved February 26, 2010.
- ↑ "Iranian agents in Kenya planned attacks on Israel, US, UK, Saudi Arabian targets". Washington Post. July 2, 2012. Retrieved July 2, 2012.
- ↑ Ronen Bergman (February 10, 2015). "The Hezbollah Connection". New York Times. Retrieved February 16, 2015.
- ↑ Annotated Reference Outline for the Toxicological Review of hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). U.S. Environmental Protection Agency (November 23, 2010)
- ↑ Schneider, N. R.; Bradley, S. L.; Andersen, M. E. (July 1976), Toxicology of cyclotrimethylenetrinitramine (RDX): Distribution and metabolism in the rat and the miniature swine, Scientific Report, DTIC, SR76-34; also in Toxicology and Applied Pharmacology 39(3) March 1977, doi:10.1016/0041-008X(77)90144-2
- ↑ Faust, Rosmarie A. (December 1994) Toxicity summary for hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX). Oak Ridge National Laboratory
- ↑ Muhly, Robert L. (December 2001) Update on the Reevaluation of the Carcinogenic Potential of RDX. U.S. Army Center for Health Promotion and Preventive Medicine (CHPPM) "white paper"
- ↑ "Hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX) (CASRN 121-82-4)". epa.gov. Retrieved January 1, 2014.
- ↑ Newell, Charles (August 2008). Treatment of RDX & HMX Plumes Using Mulch Biowalls. GSI Environmental, Inc.
- ↑ Hawari, J.; Beaudet, S.; Halasz, A.; Thiboutot, S.; Ampleman, G. (2000). "Microbial degradation of explosives: biotransformation versus mineralization". Applied Microbiology and Biotechnology 54 (5): 605–618. doi:10.1007/s002530000445. PMID 11131384.
- ↑ Panz, K.; Miksch, K. (December 2012). "Phytoremediation of explosives (TNT, RDX, HMX) by wild-type and transgenic plants". Journal of Environmental Management 113: 85–92. doi:10.1016/j.jenvman.2012.08.016. PMID 22996005.
Bibliography
- Baxter III, James Phinney (1968) [1946], Scientists Against Time (MIT Paperback ed.), Cambridge, MA: MIT Press, ISBN 978-0-262-52012-6, OCLC 476611116
- Elderfield, Robert C. (1960), Werner Emanual Bachmann: 1901–1951 (PDF), Washington DC: National Academy of Sciences
- MacDonald and Mack Partnership (August 1984), Final Properties Report: Newport Army Ammunition Plant (PDF), National Park Service, AD-A175 818
- Urbański, Tadeusz (1967), Laverton, Silvia, ed., Chemistry and Technology of Explosives III, translated by Marian Jureck (First English ed.), Warszawa: PWN – Polish Scientific Publishers and Pergamon Press, OCLC 499857211. See also ISBN 978-0-08-010401-0.
- Urbański translation https://openlibrary.org/books/OL3160546M/Chemistry_and_technology_of_explosives, Macmillan, NY, 1964, ISBN 0-08-026206-6.
Further reading
- Agrawal, Jai Prakhash.; Hodgson, Robert Dale (2007), Organic Chemistry of Explosives, Wiley, ISBN 978-0-470-02967-1
- US 2680671, Bachmann, Werner E., "Method of Treating Cyclonite Mixtures", published July 16, 1943, issued June 8, 1954
- US 2798870, Bachmann, Werner E., "Method for Preparing Explosives", published July 16, 1943, issued July 9, 1957
- Cooper, Paul W. (1996), Explosives Engineering, New York: Wiley-VCH, ISBN 0-471-18636-8
- Hale, George C. (1925), "The Nitration of Hexamethylenetetramine", Journal of the American Chemical Society 47 (11): 2754–2763, doi:10.1021/ja01688a017
- Meyer, Rudolf (1987), Explosives (3rd ed.), VCH Publishers, ISBN 0-89573-600-4
External links
- ADI Limited (Australia). Archive.org leads to Thales group products page that shows some military specifications.
- NLM Hazardous Substances Databank (US) – Cyclonite (RDX)
- CDC – NIOSH Pocket Guide to Chemical Hazards
- GlobalSecurity.org, Explosives – Compositions, Alexandria, VA: GlobalSecurity.org, retrieved September 1, 2010
- http://nla.gov.au/nla.news-article38338874, Army News (Darwin, NT), October 2, 1943, p 3. "Britain's New Explosive: Experts Killed in Terrific Blast", uses "Research Department formula X"
- http://nla.gov.au/nla.news-article42015565, The Courier-Mail (Brisbane, Qld.), September 27, 1943, p 1.
|