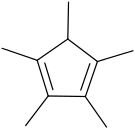
Pentamethylcyclopentadiene
 | |
 | |
| Identifiers | |
|---|---|
| 4045-44-7 | |
| ChemSpider | 70069 |
| Jmol interactive 3D | Image |
| PubChem | 77667 |
| |
| |
| Properties | |
| C10H16 | |
| Molar mass | 136.24 g/mol |
| Boiling point | 55 to 60 °C (131 to 140 °F; 328 to 333 K) (13 mm Hg) |
| Sparingly soluble | |
| Hazards | |
| Flash point | 114 °C (237 °F; 387 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic diolefin with the formula C5Me5H (Me = CH3).[1] 1,2,3,4,5-Pentamethylcyclopentadiene is the precursor to the ligand 1,2,3,4,5-pentamethylcyclopentadienyl, which is often denoted as Cp* (to signify the five methyl groups radiating from the periphery of this ligand as in a five-pointed star). In contrast to less substituted cyclopentadiene derivatives, Cp*H is not prone to dimerization.
Synthesis
Pentamethylcyclopentadiene is commercially available. It was first prepared from tiglaldehyde via 2,3,4,5-tetramethylcyclopent-2-enone.[2] Alternatively 2-butenyllithium adds to ethylacetate followed by acid-catalyzed dehydrocyclization:[3][4]
- 2 MeCH=C(Li)Me + MeC(O)OEt → (MeCH=C(Me))2C(OLi)Me + LiOEt
- (MeCH=C(Me))2C(OLi)Me + H+ → Cp*H + H2O + Li+
Organometallic derivatives
Cp*H is a precursor to organometallic compounds containing the C5Me5− (Cp*−) ligand.[5]
| Cp*-metal Complexes | |
|---|---|
| Cp*2Fe | yellow |
| Cp*TiCl3 | red |
| [Cp*Fe(CO)2]2 | red-violet |
| [Cp*RhCl2]2 | red |
| Cp*IrCl22 | orange |
| Cp*Re(CO)3 | colorless |
| Cp*Mo(CO)2CH3 | orange |
Some representative reactions leading to such Cp*-metal complexes follow:[6]
- 2 Cp*H + 2 Fe(CO)5 → [Cp*Fe(CO)2]2 + H2 + CO
For the related Cp complex, see cyclopentadienyliron dicarbonyl dimer.
An instructive but obsolete route to Cp* complexes involves the use of hexamethyl Dewar benzene. This method was traditionally used for preparation of the chloro-bridged dimers [Cp*IrCl2]2 and [Cp*RhCl2]2. Such syntheses rely on a hydrohalic acid induced rearrangement of hexamethyl Dewar benzene[7][8] to a substituted pentamethylcyclopentadiene prior to reaction with the hydrate of either iridium(III) chloride[9] or rhodium(III) chloride.[10]
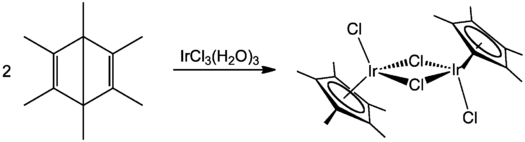
Comparison of Cp* with Cp
Complexes of pentamethylcyclopentadienyl differ in several ways from the more common cyclopentadienyl (Cp) derivatives. Being more electron-rich, Cp* is a stronger donor and is less easily removed from the metal. Consequently, its complexes exhibit increased thermal stability. Its steric bulk allows the isolation of complexes with fragile ligands. Its bulk also attenuates intermolecular interactions, decreasing the tendency to form polymeric structures. Its complexes also tend to be highly soluble in non-polar solvents. The methyl group in Cp* complexes can undergo C-H activation leading to "tuck-in complexes".
See also
References
- ↑ Overview of Cp* Compounds: Elschenbroich, C. and Salzer, A. Organometallics: a Concise Introduction (1989) p. 47
- ↑ L. de Vries (1960). "Preparation of 1,2,3,4,5-Pentamethyl-cyclopentadiene, 1,2,3,4,5,5-Hexamethyl-cyclopentadiene, and 1,2,3,4,5-Pentamethyl-cyclopentadienylcarbinol". J. Org. Chem. 25 (10): 1838. doi:10.1021/jo01080a623.
- ↑ S. Threlkel, J. E. Bercaw, P. F. Seidler, J. M. Stryker, R. G. Bergman (1993). "1,2,3,4,5-Pentamethylcyclopentadiene". Org. Synth.; Coll. Vol. 8, p. 505
- ↑ Fendrick, C. M.; Schertz, L. D.; Mintz, E. A.; Marks, T. J. (1992). "Large-Scale Synthesis of 1,2,3,4,5-Pentamethylcyclopentadiene". Inorganic Syntheses. Inorganic Syntheses 29: 193–198. doi:10.1002/9780470132609.ch47. ISBN 978-0-470-13260-9.
- ↑ Yamamoto, A. Organotransition Metal Chemistry: Fundamental Concepts and Applications. (1986) p. 105
- ↑ R. B. King, M. B. Bisnette (1967). "Organometallic chemistry of the transition metals XXI. Some π-pentamethylcyclopentadienyl derivatives of various transition metals". Journal of Organometallic Chemistry 8 (2): 287–297. doi:10.1016/S0022-328X(00)91042-8.
- ↑ Paquette, L. A.; Krow, G. R. (1968). "Electrophilic Additions to Hexamethyldewarbenzene". Tetrahedron Lett. 9 (17): 2139–2142. doi:10.1016/S0040-4039(00)89761-0.
- ↑ Criegee, R.; Gruner, H. (1968). "Acid-catalyzed Rearrangements of Hexamethyl-prismane and Hexamethyl-Dewar-benzene". Angew. Chem. Int. Ed. Engl. 7 (6): 467–468. doi:10.1002/anie.196804672.
- ↑ Kang, J. W.; Mosley, K.; Maitlis, P. M. (1968). "Mechanisms of Reactions of Dewar Hexamethylbenzene with Rhodium and Iridium Chlorides". Chem. Commun. (21): 1304–1305. doi:10.1039/C19680001304.
- ↑ Kang, J. W.; Maitlis, P. M. (1968). "Conversion of Dewar Hexamethylbenzene to Pentamethylcyclopentadienylrhodium(III) Chloride". J. Amer. Chem. Soc. 90 (12): 3259–3261. doi:10.1021/ja01014a063.