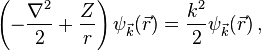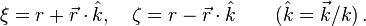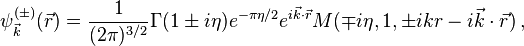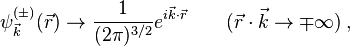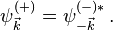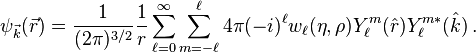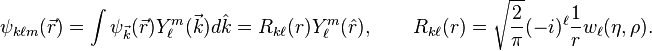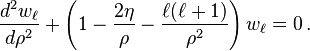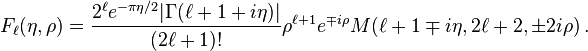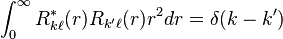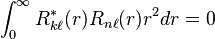Coulomb wave function
In mathematics, a Coulomb wave function is a solution of the Coulomb wave equation, named after Charles-Augustin de Coulomb. They are used to describe the behavior of charged particles in a Coulomb potential and can be written in terms of confluent hypergeometric functions or Whittaker functions of imaginary argument.
Coulomb wave equation
The Coulomb wave equation for a single charged particle is the Schrödinger equation with Coulomb potential[1]
where  is the product of the charges of the particle and of the field source (in units of the elementary charge,
is the product of the charges of the particle and of the field source (in units of the elementary charge, 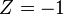 for hydrogen atom) and
for hydrogen atom) and 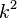 is proportional to the asymptotic energy of the particle. The solution – Coulomb wave function – can be found by solving this equation in parabolic coordinates
is proportional to the asymptotic energy of the particle. The solution – Coulomb wave function – can be found by solving this equation in parabolic coordinates
Depending on the boundary conditions chosen the solution has different forms. Two of the solutions are[2]
where  is the confluent hypergeometric function,
is the confluent hypergeometric function, 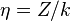 and
and  is the gamma function. The two boundary conditions used here are
is the gamma function. The two boundary conditions used here are
which correspond to  -oriented plane-wave asymptotic state before or after its approach of the field source at the origin, respectively. The functions
-oriented plane-wave asymptotic state before or after its approach of the field source at the origin, respectively. The functions 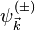 are related to each other by the formula
are related to each other by the formula
Partial wave expansion
The wave function 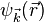 can be expanded into partial waves (i.e. with respect to the angular basis) to obtain angle-independent radial functions
can be expanded into partial waves (i.e. with respect to the angular basis) to obtain angle-independent radial functions 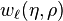 . Here
. Here 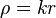 .
.
A single term of the expansion can be isolated by the scalar product with a specific angular state
\sqrt{\frac{2}{\pi}} part seems off, wrong norm factor, orthonormality down below not true like this. Please check it. The equation for single partial wave 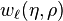 can be obtained by rewriting the laplacian in the Coulomb wave equation in spherical coordinates and projecting the equation on a specific spherical harmonic
can be obtained by rewriting the laplacian in the Coulomb wave equation in spherical coordinates and projecting the equation on a specific spherical harmonic 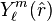
The solutions are also called Coulomb (partial) wave functions. Putting 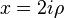 changes the Coulomb wave equation into the Whittaker equation, so Coulomb wave functions can be expressed in terms of Whittaker functions with imaginary arguments.
Two special solutions called the regular and irregular Coulomb wave functions are denoted by
changes the Coulomb wave equation into the Whittaker equation, so Coulomb wave functions can be expressed in terms of Whittaker functions with imaginary arguments.
Two special solutions called the regular and irregular Coulomb wave functions are denoted by 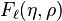 and
and 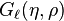 , and defined in terms of the confluent hypergeometric function by[3][4]
, and defined in terms of the confluent hypergeometric function by[3][4]
The two possible sets of signs are related to each other by the Kummer transform.
Properties of the Coulomb function
The radial parts for a given angular momentum are orthonormal,[5]
and for 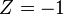 they are also orthogonal to all hydrogen bound states[6]
they are also orthogonal to all hydrogen bound states[6]
due to being eigenstates of the same hermitian operator (the hamiltonian) with different eigenvalues.
Further reading
- Bateman, Harry (1953), Higher transcendental functions (PDF) 1, McGraw-Hill.
- Jaeger, J. C.; Hulme, H. R. (1935), "The Internal Conversion of γ -Rays with the Production of Electrons and Positrons", Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences (The Royal Society) 148 (865): 708–728, Bibcode:1935RSPSA.148..708J, doi:10.1098/rspa.1935.0043, ISSN 0080-4630, JSTOR 96298
- Slater, Lucy Joan (1960), Confluent hypergeometric functions, Cambridge University Press, MR 0107026.
References
- ↑ Hill, Robert N. (2006), Drake, Gordon, ed., Handbook of atomic, molecular and optical physics, Springer New York, pp. 153–155
- ↑ Landau, L. D.; Lifshitz, E. M. (1977), Course of theoretical physics III: Quantum mechanics, Non-relativistic theory (3rd ed.), Pergamon Press, p. 569
- ↑ Thompson, I. J. (2010), "Coulomb Functions", in Olver, Frank W. J.; Lozier, Daniel M.; Boisvert, Ronald F.; Clark, Charles W., NIST Handbook of Mathematical Functions, Cambridge University Press, ISBN 978-0521192255, MR 2723248
- ↑ Abramowitz, Milton; Stegun, Irene A., eds. (December 1972) [1964]. "Chapter 14". Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables. Applied Mathematics Series 55 (10 ed.). New York, USA: United States Department of Commerce, National Bureau of Standards; Dover Publications. p. 538. ISBN 978-0-486-61272-0. LCCN 64-60036. MR 0167642.
- ↑ Formánek, Jiří (2004), Introduction to quantum theory I (in Czech) (2nd ed.), Prague: Academia, pp. 128–130
- ↑ Landau, L. D.; Lifshitz, E. M. (1977), Course of theoretical physics III: Quantum mechanics, Non-relativistic theory (3rd ed.), Pergamon Press, pp. 668–669