Coral
| Coral | |
|---|---|
_(San_Salvador_Island%2C_Bahamas)_1_(15513345363).jpg) | |
| Pillar coral, Dendrogyra cylindricus | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Cnidaria |
| Class: | Anthozoa Ehrenberg, 1831 |
| Extant Subclasses and Orders | |
|
Octocorallia [1] | |
Corals are marine invertebrates in the class Anthozoa of phylum Cnidaria. They typically live in compact colonies of many identical individual polyps. The group includes the important reef builders that inhabit tropical oceans and secrete calcium carbonate to form a hard skeleton.
A coral "group" is a colony of myriad genetically identical polyps. Each polyp is a sac-like animal typically only a few millimeters in diameter and a few centimeters in length. A set of tentacles surround a central mouth opening. An exoskeleton is excreted near the base. Over many generations, the colony thus creates a large skeleton that is characteristic of the species. Individual heads grow by asexual reproduction of polyps. Corals also breed sexually by spawning: polyps of the same species release gametes simultaneously over a period of one to several nights around a full moon.
Although some corals can catch small fish and plankton, using stinging cells on their tentacles, most corals obtain the majority of their energy and nutrients from photosynthetic unicellular dinoflagellates in the genus Symbiodinium that live within their tissues. These are commonly known as zooxanthellae and the corals that contain them are zooxanthellate corals. Such corals require sunlight and grow in clear, shallow water, typically at depths shallower than 60 metres (200 ft). Corals are major contributors to the physical structure of the coral reefs that develop in tropical and subtropical waters, such as the enormous Great Barrier Reef off the coast of Queensland, Australia.
Other corals do not rely on zooxanthellae and can live in much deeper water, with the cold-water genus Lophelia surviving as deep as 3,000 metres (9,800 ft).[3] Some have been found on the Darwin Mounds, north-west of Cape Wrath, Scotland. Corals have also been found as far north as off the coast of Washington State and the Aleutian Islands.
Taxonomy
| |||||||||||||||||||||||||||||||||||||||
| Phylogeny of Anthozoa |
In his Scala Naturae, Aristotle classified corals as "zoophyta" ("plant-animals"), animals that had characteristics of plants and were therefore hypothetically in between animals and plants. The Persian polymath Al-Biruni (d. 1048) classified sponges and corals as animals, arguing that they respond to touch.[4] Nevertheless, people believed corals to be plants until the eighteenth century, when William Herschel used a microscope to establish that coral had the characteristic thin cell membranes of an animal.[5]
The phylogeny of Anthozoans is not clearly understood and a number of different models have been proposed. Within the Hexacorallia, the sea anemones, coral anemones and stony corals may constitute a monophyletic grouping united by their eight-fold symmetry and cnidocyte trait. The Octocorallia appears to be monophyletic, and primitive members of this group may have been stolonate.[6] The cladogram presented here comes from a 2014 study by Stampar et al. which was based on the divergence of mitochondrial DNA within the group and on nuclear markers.[7]
Corals are classified in the class Anthozoa of the phylum Cnidaria. They are divided into three subclasses, Hexacorallia, Octocorallia,[8] and Ceriantharia.[7][9] The Hexacorallia include the stony corals, the sea anemones and the zoanthids. These groups have polyps that generally have 6-fold symmetry. The Octocorallia include blue coral, soft corals, sea pens, and gorgonians (sea fans and sea whips). These groups have polyps with 8-fold symmetry, each polyp having eight tentacles and eight mesenteries. Ceriantharia are the tube-dwelling anemones.[6]
Fire corals are not true corals, being in the order Anthomedusa (sometimes known as Anthoathecata) of the class Hydrozoa.[10]
Anatomy

Corals are sessile animals in the class Anthozoa and differ from most other cnidarians in not having a medusa stage in their life cycle. The body unit of the animal is a polyp. Most corals are colonial, the initial polyp budding to produce another and the colony gradually developing from this small start. In stony corals, also known as hard corals, the polyps produce a skeleton composed of calcium carbonate to strengthen and protect the organism. This is deposited by the polyps and by the coenosarc, the living tissue that connects them. The polyps sit in cup-shaped depressions in the skeleton known as corallites. Colonies of stony coral are very variable in appearance; a single species may adopt an encrusting, plate-like, bushy, columnar or massive solid structure, the various forms often being linked to different types of habitat, with variations in light level and water movement being significant.[6]
In soft corals, there is no stony skeleton but the tissues are often toughened by the presence of tiny skeletal elements known as sclerites, which are made from calcium carbonate. Soft corals are very variable in form and most are colonial. A few soft corals are stolonate, but the polyps of most are connected by sheets of coenosarc. In some species this is thick and the polyps are deeply embedded. Some soft corals are encrusting or form lobes. Others are tree-like or whip-like and have a central axial skeleton embedded in the tissue matrix. This is composed either of a fibrous protein called gorgonin or of a calcified material. In both stony and soft corals, the polyps can be retracted, with stony corals relying on their hard skeleton and cnidocytes for defence against predators, with soft corals generally relying on chemical defences in the form of toxic substances present in the tissues known as terpenoids.[6]

The polyps of stony corals have six-fold symmetry while those of soft corals have eight. The mouth of each polyp is surrounded by a ring of tentacles. In stony corals these are cylindrical and taper to a point, but in soft corals they are pinnate with side branches known as pinnules. In some tropical species these are reduced to mere stubs and in some they are fused to give a paddle-like appearance.[11] In most corals, the tentacles are retracted by day and spread out at night to catch plankton and other small organisms. Shallow water species of both stony and soft corals can be zooxanthellate, the corals supplementing their plankton diet with the products of photosynthesis produced by these symbionts.[6] The polyps interconnect by a complex and well-developed system of gastrovascular canals, allowing significant sharing of nutrients and symbionts.[12]
Ecology
Feeding
Polyps feed on a variety of small organisms, from microscopic zooplankton to small fish. The polyp's tentacles immobilize or kill prey using their nematocysts. These cells carry venom which they rapidly release in response to contact with another organism. A dormant nematocyst discharges in response to nearby prey touching the trigger (cnidocil). A flap (operculum) opens, and its stinging apparatus fires the barb into the prey. The venom is injected through the hollow filament to immobilise the prey; the tentacles then manoeuvre the prey to the mouth.[13]
The tentacles then contract to bring the prey into the stomach. Once the prey is digested, the stomach reopens, allowing the elimination of waste products and the beginning of the next hunting cycle. They can scavenge drifting organic molecules and dissolved organic molecules.[14]:24
Intracellular symbionts
Many corals, as well as other cnidarian groups such as Aiptasia (a sea anemone) form a symbiotic relationship with a class of dinoflagellate algae, zooxanthellae of the genus Symbiodinium.[14]:24 Aiptasia, a familiar pest among coral reef aquarium hobbyists, serves as a valuable model organism in the study of cnidarian-algal symbiosis. Typically, each polyp harbors one species of algae. Via photosynthesis, these provide energy for the coral, and aid in calcification.[15] As much as 30% of the tissue of a polyp may be plant material.[14]:23
The algae benefit from a safe place to live and consume the polyp's carbon dioxide and nitrogenous waste. Due to the strain the algae can put on the polyp, stress on the coral often drives them to eject the algae. Mass ejections are known as coral bleaching, because the algae contribute to coral's brown coloration; other colors, however, are due to host coral pigments, such as green fluorescent proteins (GFPs). Ejection increases the polyp's chance of surviving short-term stress—they can regain algae, possibly of a different species at a later time. If the stressful conditions persist, the polyp eventually dies.[16]
Reproduction
Corals can be both gonochoristic (unisexual) and hermaphroditic, each of which can reproduce sexually and asexually. Reproduction also allows coral to settle in new areas.
Sexual
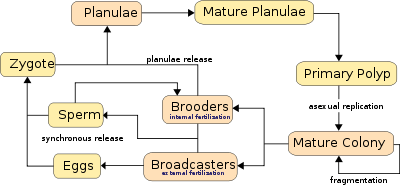
Corals predominantly reproduce sexually. About 25% of hermatypic corals (stony corals) form single sex (gonochoristic) colonies, while the rest are hermaphroditic.[17]
Broadcasters
About 75% of all hermatypic corals "broadcast spawn" by releasing gametes—eggs and sperm—into the water to spread offspring. The gametes fuse during fertilization to form a microscopic larva called a planula, typically pink and elliptical in shape. A typical coral colony forms several thousand larvae per year to overcome the odds against formation of a new colony.[18]

Synchronous spawning is very typical on the coral reef, and often, even when multiple species are present, all corals spawn on the same night. This synchrony is essential so male and female gametes can meet. Corals rely on environmental cues, varying from species to species, to determine the proper time to release gametes into the water. The cues involve temperature change, lunar cycle, day length, and possibly chemical signalling.[17] Synchronous spawning may form hybrids and is perhaps involved in coral speciation.[19] The immediate cue is most often sunset, which cues the release.[17] The spawning event can be visually dramatic, clouding the usually clear water with gametes.
Brooders
Brooding species are most often ahermatypic (not reef-building) in areas of high current or wave action. Brooders release only sperm, which is negatively buoyant, sinking on to the waiting egg carriers who harbor unfertilized eggs for weeks. Synchronous spawning events sometimes occurs even with these species.[17] After fertilization, the corals release planula that are ready to settle.[15]
Planulae
Planulae exhibit positive phototaxis, swimming towards light to reach surface waters, where they drift and grow before descending to seek a hard surface to which they can attach and begin a new colony. They also exhibit positive sonotaxis, moving towards sounds that emanate from the reef and away from open water.[20] High failure rates afflict many stages of this process, and even though millions of gametes are released by each colony, few new colonies form. The time from spawning to settling is usually two to three days, but can be up to two months.[21] The larva grows into a polyp and eventually becomes a coral head by asexual budding and growth.
Asexual

Within a coral head, the genetically identical polyps reproduce asexually, either by budding (gemmation) or by dividing, whether longitudinally or transversely.
Budding involves splitting a smaller polyp from an adult.[18] As the new polyp grows, it forms its body parts. The distance between the new and adult polyps grows, and with it, the coenosarc (the common body of the colony; see coral anatomy). Budding can be intratentacular, from its oral discs, producing same-sized polyps within the ring of tentacles, or extratentacular, from its base, producing a smaller polyp.
Division forms two polyps that each become as large as the original. Longitudinal division begins when a polyp broadens and then divides its coelenteron (body), effectively splitting along its length. The mouth divides and new tentacles form. The two polyps thus created then generate their missing body parts and exoskeleton. Transversal division occurs when polyps and the exoskeleton divide transversally into two parts. This means one has the basal disc (bottom) and the other has the oral disc (top); the new polyps must separately generate the missing pieces.
Asexual reproduction offers the benefts of high reproductive rate, delaying senescence, and replacement of dead modules, as well as geographical distribution.[22]
Colony division
Whole colonies can reproduce asexually, forming two colonies with the same genotype. The possible mechanisms include fission, bailout and fragmentation. Fission occurs in some corals, especially among the family Fungiidae, where the colony splits into two or more colonies during early developmental stages. Bailout occurs when a single polyp abandons the colony and settles on a different substrate to create a new colony. Fragmentation involves individuals broken from the colony during storms or other disruptions. The separated individuals can start new colonies.[23]
Reefs

Many corals in the order Scleractinia are hermatypic, meaning that they are involved in building reefs. Most such corals obtain some of their energy from zooxanthellae in the genus Symbiodinium. These are symbiotic photosynthetic dinoflagellates which require sunlight; reef-forming corals are therefore found mainly in shallow water. They secrete calcium carbonate to form hard skeletons that become the framework of the reef. However, not all reef-building corals in shallow water contain zooxanthellae, and some deep water species, living at depths to which light cannot penetrate, form reefs but do not harbour the symbionts.[24]

There are various types of shallow-water coral reef, including fringing reefs, barrier reefs and atolls; most occur in tropical and subtropical seas. They are very slow-growing, adding perhaps one centimetre (0.4 in) in height each year. The Great Barrier Reef is thought to have been laid down about two million years ago. Over time, corals fragment and die, sand and rubble accumulates between the corals, and the shells of clams and other molluscs decay to form a gradually evolving calcium carbonate structure.[25] Coral reefs are extremely diverse marine ecosystems hosting over 4,000 species of fish, massive numbers of cnidarians, molluscs, crustaceans, and many other animals.[26]
Evolutionary history

Although corals first appeared in the Cambrian period,[27] some 542 million years ago, fossils are extremely rare until the Ordovician period, 100 million years later, when rugose and tabulate corals became widespread. Paleozoic corals often contained numerous endobiotic symbionts.[28][29]
Tabulate corals occur in limestones and calcareous shales of the Ordovician and Silurian periods, and often form low cushions or branching masses of calcite alongside rugose corals. Their numbers began to decline during the middle of the Silurian period, and they became extinct at the end of the Permian period, 250 million years ago.[30]
Rugose or horn corals became dominant by the middle of the Silurian period, and became extinct early in the Triassic period. The rugose corals existed in solitary and colonial forms, and were also composed of calcite.[31]
The scleractinian corals filled the niche vacated by the extinct rugose and tabulate species. Their fossils may be found in small numbers in rocks from the Triassic period, and became common in the Jurassic and later periods.[32] Scleractinian skeletons are composed of a form of calcium carbonate known as aragonite.[33] Although they are geologically younger than the tabulate and rugose corals, the aragonite of their skeletons is less readily preserved, and their fossil record is accordingly less complete.
 | |
|
Timeline of the major coral fossil record and developments from 650 m.y.a. to present.[34][35] |
|
At certain times in the geological past, corals were very abundant. Like modern corals, these ancestors built reefs, some of which ended as great structures in sedimentary rocks. Fossils of fellow reef-dwellers algae, sponges, and the remains of many echinoids, brachiopods, bivalves, gastropods, and trilobites appear along with coral fossils. This makes some corals useful index fossils.[36] Coral fossils are not restricted to reef remnants, and many solitary fossils may be found elsewhere, such as Cyclocyathus, which occurs in England's Gault clay formation.
Status
Threats

Coral reefs are under stress around the world.[37] In particular, coral mining, agricultural and urban runoff, pollution (organic and inorganic), overfishing, blast fishing, disease, and the digging of canals and access into islands and bays are localized threats to coral ecosystems. Broader threats are sea temperature rise, sea level rise and pH changes from ocean acidification, all associated with greenhouse gas emissions.[38] In 1998, 16% of the world's reefs died as a result of increased water temperature.[39]
Approximately 10% of the world's coral reefs are dead.[40][41][42] About 60% of the world's reefs are at risk due to human-related activities.[43] The threat to reef health is particularly strong in Southeast Asia, where 80% of reefs are endangered.[44] Over 50% of the world's coral reefs may be destroyed by 2030; as a result, most nations protect them through environmental laws.[45]
In the Caribbean and tropical Pacific, direct contact between ~40–70% of common seaweeds and coral causes bleaching and death to the coral via transfer of lipid-soluble metabolites.[46] Seaweed and algae proliferate given adequate nutrients and limited grazing by herbivores such as parrotfish.
Water temperature changes of more than 1–2 °C (1.8–3.6 °F) or salinity changes can kill some species of coral. Under such environmental stresses, corals expel their Symbiodinium; without them coral tissues reveal the white of their skeletons, an event known as coral bleaching.[47]
Submarine springs found along the coast of Mexico's Yucatán Peninsula produce water with a naturally low pH (relatively high acidity) providing conditions similar to those expected to become widespread as the oceans absorb carbon dioxide.[48] Surveys discovered multiple species of live coral that appeared to tolerate the acidity. The colonies were small and patchily distributed, and had not formed structurally complex reefs such as those that compose the nearby Mesoamerican Barrier Reef System.[48]
Protection

Marine Protected Areas (MPAs), Biosphere reserves, marine parks, national monuments world heritage status, fishery management and habitat protection can protect reefs from anthropogenic damage.[49]
Many governments now prohibit removal of coral from reefs, and inform coastal residents about reef protection and ecology. While local action such as habitat restoration and herbivore protection can reduce local damage, the longer-term threats of acidification, temperature change and sea-level rise remain a challenge.[38]
To eliminate destruction of corals in their indigenous regions, projects have been started to grow corals in non-tropical countries.[50][51]
Relation to humans
Local economies near major coral reefs benefit from an abundance of fish and other marine creatures as a food source. Reefs also provide recreational scuba diving and snorkeling tourism. These activities can damage coral but international projects such as Green Fins that encourage dive and snorkel centres to follow a Code of Conduct have been proven to mitigate these risks.[52]
Live coral is highly sought after for aquaria. Soft corals are easier to maintain in captivity than hard corals.[53]
Jewelry
%2C_ca._1920s%2C_71.57.1.jpg)
Corals' many colors give it appeal for necklaces and other jewelry. Intensely red coral is prized as a gemstone. Sometimes called fire coral, it is not the same as fire coral. Red coral is very rare because of overharvesting.[54]
Medicine
In medicine, chemical compounds from corals are used for cancer, AIDS, pain, and other uses. Coral skeletons, e.g. Isididae are also used for bone grafting in humans.[55] Coral Calx, known as Praval Bhasma in Sanskrit, is widely used in traditional system of Indian medicine as a supplement in the treatment of a variety of bone metabolic disorders associated with calcium deficiency.[56]
Construction

Coral reefs in places such as the East African coast are used as a source of building material.[57] Ancient (fossil) coral limestone, notably including the Coral Rag Formation of the hills around Oxford (England), was once used as a building stone, and can be seen in some of the oldest buildings in that city including the Saxon tower of St Michael at the Northgate, St. George's Tower of Oxford Castle, and the mediaeval walls of the city.[58]
Climate research
Annual growth bands in some corals, such as the deep sea bamboo corals (Isididae), may be among the first signs of the effects of ocean acidification on marine life.[59] The growth rings allow geologists to construct year-by-year chronologies, a form of incremental dating, which underlie high-resolution records of past climatic and environmental changes using geochemical techniques.[60]
Certain species form communities called microatolls, which are colonies whose top is dead and mostly above the water line, but whose perimeter is mostly submerged and alive. Average tide level limits their height. By analyzing the various growth morphologies, microatolls offer a low resolution record of sea level change. Fossilized microatolls can also be dated using Radiocarbon dating. Such methods can help to reconstruct Holocene sea levels.[61]
Increasing sea temperatures in tropical regions (~1 degree C) the last century have caused major coral bleaching, death, and therefore shrinking coral populations since although they are able to adapt and acclimate, it is uncertain if this evolutionary process will happen quickly enough to prevent major reduction of their numbers.[62]
Though coral have large sexually-reproducing populations, their evolution can be slowed by abundant asexual reproduction.[63] Gene flow is variable among coral species.[63] According to the biogeography of coral species gene flow cannot be counted on as a dependable source of adaptation as they are very stationary organisms. Also, coral longevity might factor into their adaptivity.[63]
However, adaptation to climate changes has been demonstrated in many cases. These are usually due to a shift in coral and zooxanthellae genotypes. These shifts in allelic frequencies have progressed toward more tolerant types of zooxanthellae.[64] Scientists found that a certain scleractinian zooxanthella is becoming more common where sea temperature is high.[65][66] Symbionts able to tolerate warmer water seem to photosynthesise more slowly, implying an evolutionary trade-off.[66]
In the Gulf of Mexico, where sea temperatures are rising, cold-sensitive staghorn and elkhorn coral have shifted in location.[64] Not only have the symbionts and specific species been shown to shift, but there seems to be a certain growth rate favorable to selection. Slower-growing but more heat-tolerant corals have become more common.[67] The changes in temperature and acclimation are complex. Some reefs in current shadows represent a refugium location that will help them adjust to the disparity in the environment even if eventually the temperatures may rise more quickly there than in other locations.[68] This separation of populations by climatic barriers causes a realized niche to shrink greatly in comparison to the old fundamental niche.
Geochemistry
Corals are shallow, colonial organisms that integrate δ18O and trace elements into their skeletal aragonite (polymorph of calcite) crystalline structures, as they grow. Geochemistry anomalies within the crystalline structures of corals represent functions of temperature, salinity and oxygen isotopic composition. Such geochemical analysis can help with climate modeling.[69]
Strontium/calcium ratio anomaly
Time can be attributed to coral geochemistry anomalies by correlating strontium/calcium minimums with sea surface temperature (SST) maximums to data collected from NINO 3.4 SSTA.[70]
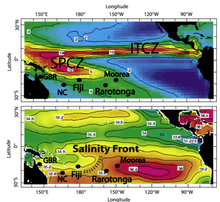
Oxygen isotope anomaly
The comparison of coral strontium/calcium minimums with sea surface temperature maximums, data recorded from NINO 3.4 SSTA, time can be correlated to coral strontium/calcium and δ18O variations. To confirm accuracy of the annual relationship between Sr/Ca and δ18O variations, a perceptible association to annual coral growth rings confirms the age conversion. Geochronology is established by the blending of Sr/Ca data, growth rings, and stable isotope data. El Nino-Southern Oscillation (ENSO) is directly related to climate fluctuations that influence coral δ18O ratio from local salinity variations associated with the position of the South Pacific convergence zone (SPCZ) and can be used for ENSO modeling.[70]
Sea surface temperature and sea surface salinity
The global moisture budget is primarily being influenced by tropical sea surface temperatures from the position of the Intertropical Convergence Zone (ITCZ).[71] The Southern Hemisphere has a unique meteorological feature positioned in the southwestern Pacific Basin called the South Pacific Convergence Zone (SPCZ), which contains a perennial position within the Southern Hemisphere. During ENSO warm periods, the SPCZ reverses orientation extending from the equator down south through Solomon Islands, Vanuatu, Fiji and towards the French Polynesian Islands; and due east towards South America affecting geochemistry of corals in tropical regions.[72]
Geochemical analysis of skeletal coral can be linked to sea surface salinity (SSS) and sea surface temperature (SST), from El Nino 3.4 SSTA data, of tropical oceans to seawater δ18O ratio anomalies from corals. ENSO phenomenon can be related to variations in sea surface salinity (SSS) and sea surface temperature (SST) that can help model tropical climate activities.[73]
Limited climate research on current species

Climate research on live coral species is limited to a few studied species. Studying Porites coral provides a stable foundation for geochemical interpretations that is much simpler to physically extract data in comparison to Platygyra species where the complexity of Platygyra species skeletal structure creates difficulty when physically sampled, which happens to be one of the only multidecadal living coral records used for coral paleoclimate modeling.[73]
Aquaria

The saltwater fishkeeping hobby has increasingly expanded, over recent years, to include reef tanks, fish tanks that include large amounts of live rock on which coral is allowed to grow and spread.[74] These tanks are either kept in a natural-like state, with algae (sometimes in the form of an algae scrubber) and a deep sand bed providing filtration,[75] or as "show tanks", with the rock kept largely bare of the algae and microfauna that would normally populate it,[76] in order to appear neat and clean.
The most popular kind of coral kept is soft coral, especially zoanthids and mushroom corals, which are especially easy to grow and propagate in a wide variety of conditions,[77] because they originate in enclosed parts of reefs where water conditions vary and lighting may be less reliable and direct.[78] More serious fishkeepers may keep small polyp stony coral, which is from open, brightly lit reef conditions and therefore much more demanding, while large polyp stony coral is a sort of compromise between the two.
Aquaculture
Coral aquaculture, also known as coral farming or coral gardening, is the cultivation of corals for commercial purposes or coral reef restoration. Aquaculture is showing promise as a potentially effective tool for restoring coral reefs, which have been declining around the world.[79][80][81] The process bypasses the early growth stages of corals when they are most at risk of dying. Coral fragments known as "seeds" are grown in nurseries then replanted on the reef.[82] Coral is farmed by coral farmers who live locally to the reefs and farm for reef conservation or for income. It is also farmed by scientists for research, by businesses for the supply of the live and ornamental coral trade and by private aquarium hobbyists.
Gallery
Further images: commons:Category:Coral reefs and commons:Category:Coral
-
Fungia sp. skeleton
-

Polyps of Eusmilia fastigiata
-

Staghorn coral, Acropora
-
_01.jpg)
Orange cup coral, Balanophyllia elegans
-

Brain coral spawning
-

Brain coral releasing eggs
-

Fringing coral reef off the coast of Eilat, Israel.
References
- ↑ Hoeksema, Bert (2015). "Octocorallia". World Register of Marine Species. Retrieved 2015-04-24.
- ↑ Hoeksema, Bert (2015). "Hexacorallia". World Register of Marine Species. Retrieved 2015-04-24.
- ↑ Squires, D.F. (1959). "Deep sea corals collected by the Lamont Geological Observatory. 1. Atlantic corals". American Museum Novitates 1965: 1–42.
- ↑ Egerton, Frank N. (2012). Roots of Ecology: Antiquity to Haeckel. University of California Press. p. 24. ISBN 0520953630.
- ↑ The Light of Reason 8 August 2006 02:00 BBC Four
- 1 2 3 4 5 Ruppert, Edward E.; Fox, Richard, S.; Barnes, Robert D. (2004). Invertebrate Zoology, 7th edition. Cengage Learning. pp. 132–148. ISBN 978-81-315-0104-7.
- 1 2 Stampar, S.N.; Maronna, M.M.; Kitahara, M.V.; Reimer, J.D.; Morandini, A.C. (2014). "Fast-Evolving Mitochondrial DNA in Ceriantharia: A Reflection of Hexacorallia Paraphyly?". PLoS ONE 9 (1): e86612. doi:10.1371/journal.pone.0086612. PMC 3903554. PMID 24475157.
- ↑ Hoeksema, Bert (2015). "Anthozoa". World Register of Marine Species. Retrieved 2015-04-24.
- ↑ Chen, C. A.; Odorico, D. M.; ten Lohuis, M.; Veron, J. E. N.; Miller, D. J. (1995). "Systematic relationships within the Anthozoa (Cnidaria: Anthozoa) using the 5'-end of the 28S rDNA" (PDF). Molecular Phylogeny and Evolution 4 (2): 175–183. doi:10.1006/mpev.1995.1017. PMID 7663762.
- ↑ Schuchert, Peter (2015). "Milleporidae Fleming, 1828". World Register of Marine Species. Retrieved 2015-04-24.
- ↑ Sprung, Julian (1999). Corals: A quick reference guide. Ricordea Publishing. p. 145. ISBN 1-883693-09-8.
- ↑ D. Gateno, A. Israel, Y. Barki and B. Rinkevich (1998). "Gastrovascular Circulation in an Octocoral: Evidence of Significant Transport of Coral and Symbiont Cells". The Biological Bulletin (Marine Biological Laboratory) 194 (2): 178–186. doi:10.2307/1543048. JSTOR 1543048.
- ↑ "Coral Feeding Habits". NOAA. Retrieved 25 April 2015.
- 1 2 3 Murphy, Richard C. (2002). Coral Reefs: Cities Under The Seas. The Darwin Press. ISBN 0-87850-138-X.
- 1 2 Madl, P. and Yip, M. (2000). "Field Excursion to Milne Bay Province – Papua New Guinea". Retrieved 2006-03-31.
- ↑ W. W. Toller, R. Rowan and N. Knowlton (2001). "Repopulation of Zooxanthellae in the Caribbean Corals Montastraea annularis and M. faveolata following Experimental and Disease-Associated Bleaching". The Biological Bulletin (Marine Biological Laboratory) 201 (3): 360–373. doi:10.2307/1543614. JSTOR 1543614. PMID 11751248.
- 1 2 3 4 Veron, J.E.N. (2000). Corals of the World. Vol 3 (3rd ed.). Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd. ISBN 0-642-32236-8.
- 1 2 Barnes, R. and; Hughes, R. (1999). An Introduction to Marine Ecology (3rd ed.). Malden, MA: Blackwell. pp. 117–141. ISBN 0-86542-834-4.
- ↑ Hatta, M., Fukami, H., Wang, W., Omori, M., Shimoike, K., Hayashibara, T., Ina, Y., Sugiyama, T. (1999). "Reproductive and genetic evidence for a reticulate evolutionary theory of mass spawning corals" (PDF). Molecular Biology and Evolution 16 (11): 1607–1613. doi:10.1093/oxfordjournals.molbev.a026073. PMID 10555292.
- ↑ Vermeij, Mark J. A.; Marhaver, Kristen L.; Huijbers, Chantal M.; Nagelkerken, Ivan; Simpson, Stephen D. (2010). "Coral Larvae Move toward Reef Sounds". PLoS ONE 5 (5): e10660. doi:10.1371/journal.pone.0010660. PMID 20498831. Lay summary – ScienceDaily (May 16, 2010).
- ↑ Jones, O.A. and R. Endean. (1973). Biology and Geology of Coral Reefs. New York, USA: Harcourt Brace Jovanovich. pp. 205–245. ISBN 0-12-389602-9.
- ↑ Gulko, David (1998). Hawaiian Coral Reef Ecology. Honolulu, Hawaii: Mutual Publishing. p. 10. ISBN 1-56647-221-0.
- ↑ Sheppard, Charles R.C.; Davy, Simon K.; Pilling, Graham M. (25 June 2009). The Biology of Coral Reefs. OUP Oxford. pp. 78–81. ISBN 978-0-19-105734-2.
- ↑ Schuhmacher, Helmut; Zibrowius, Helmut (1985). "What is hermatypic?". Coral Reefs 4 (1): 1–9. doi:10.1007/BF00302198.
- ↑ MSN Encarta (2006). Great Barrier Reef. Archived from the original on October 31, 2009. Retrieved April 25, 2015.
- ↑ Spalding, Mark; Ravilious, Corinna; Green, Edmund (2001). World Atlas of Coral Reefs. Berkeley, CA: University of California Press and UNEP/WCMC. pp. 205–245. ISBN 0-520-23255-0.
- ↑ Pratt, B.R.; Spincer, B.R., R.A. Wood and A.Yu. Zhuravlev (2001). "12: Ecology and Evolution of Cambrian Reefs". Ecology of the Cambrian Radiation (PDF). Columbia University Press. p. 259. ISBN 0-231-10613-0. Retrieved 2007-04-06.
- ↑ Vinn, O.; Mõtus, M.-A. (2008). "The earliest endosymbiotic mineralized tubeworms from the Silurian of Podolia, Ukraine". Journal of Paleontology 82: 409–414. doi:10.1666/07-056.1. Retrieved 2014-06-11.
- ↑ Vinn, O.; Mõtus, M.-A. (2012). "Diverse early endobiotic coral symbiont assemblage from the Katian (Late Ordovician) of Baltica". Palaeogeography, Palaeoclimatology, Palaeoecology. 321–322: 137–141. doi:10.1016/j.palaeo.2012.01.028. Retrieved 2014-06-11.
- ↑ "Introduction to the Tabulata". UCMP Berkeley. Retrieved 25 April 2015.
- ↑ "Introduction to the Rugosa". UCMP Berkeley. Retrieved 25 April 2015.
- ↑ "Evolutionary history". AIMS. Retrieved 25 April 2015.
- ↑ Ries, J.B., Stanley, S.M., Hardie, L.A.; Stanley; Hardie (July 2006). "Scleractinian corals produce calcite, and grow more slowly, in artificial Cretaceous seawater". Geology 34 (7): 525–528. doi:10.1130/G22600.1.
- ↑ Ben M. Waggoner (2000). David Smith and Allen Collins, ed. "Anthozoa: Fossil Record". Anthozoa. UCMP. Retrieved 2009-03-23.
- ↑ William A. Oliver, Jr. (2003). "Corals: Table 1". Fossil Groups. USGS. Retrieved 2009-03-23.
- ↑ Alden, Andrew. "Index Fossils". About education. Retrieved 25 April 2015.
- ↑ "Coral reefs around the world". Guardian.co.uk. 2 September 2009.
- 1 2 "Threats to Coral Reefs". Coral Reef Alliance. 2010. Retrieved 5 December 2011.
- ↑ Losing Our Coral Reefs – Eco Matters – State of the Planet. Blogs.ei.columbia.edu. Retrieved on 2011-11-01.
- ↑ Kleypas, J.A.; Feely, R.A.; Fabry, V.J.; Langdon, C.; Sabine, C.L.; Robbins, L.L. (2006). "Impacts of Ocean Acidification on Coral Reefs and Other Marine Calcifiers: A guide for Future Research" (PDF). National Science Foundation, NOAA, & United States Geological Survey. Retrieved April 7, 2011.
- ↑ Save Our Seas, 1997 Summer Newsletter, Dr. Cindy Hunter and Dr. Alan Friedlander
- ↑ Tun, K.; Chou, L.M.; Cabanban, A.; Tuan, V.S.; Philreefs; Yeemin, T.; Suharsono; Sour, K.; Lane, D. (2004). "Status of Coral Reefs, Coral Reef Monitoring and Management in Southeast Asia, 2004". In Wilkinson, C. Status of Coral Reefs of the world: 2004. Townsville, Queensland, Australia: Australian Institute of Marine Science. pp. 235–276.
- ↑ Burke, Lauretta; Reytar, K.; Spalding, M.; Perry, A. (2011). Reefs at risk revisited. Washington, DC: World Resources Institute. p. 38. ISBN 978-1-56973-762-0.
- ↑ Bryant, Dirk; Burke, Lauretta; McManus, John; Spalding, Mark. "Reefs at Risk: A Map-Based Indicator of Threats to the World’s Coral Reef" (PDF). NOAA. Retrieved 25 April 2015.
- ↑ Norlander (8 December 2003). "Coral crisis! Humans are killing off these bustling underwater cities. Can coral reefs be saved? (Life science: corals)". Science World.
- ↑ Rasher DB, Hay ME; Hay (May 2010). "Chemically rich seaweeds poison corals when not controlled by herbivores". Proceedings of the National Academy of Sciences of the United States of America 107 (21): 9683–8. doi:10.1073/pnas.0912095107. PMC 2906836. PMID 20457927.
- ↑ Hoegh-Guldberg, O. (1999). "Climate change, coral bleaching and the future of the world's coral reefs" (PDF). Marine and Freshwater Research 50 (8): 839–866. doi:10.1071/MF99078.
- 1 2 Stephens, Tim (28 November 2011). "Submarine springs offer preview of ocean acidification effects on coral reefs". University of California Santa Cruz. Retrieved 25 April 2015.
- ↑ "Phoenix Rising". National Geographic Magazine. January 2011. Retrieved April 30, 2011.
- ↑ EcoDeco EcologicalTechnology. Ecodeco.nl. Retrieved on 2011-11-29.
- ↑ KoralenKAS project. Koraalwetenschap.nl. Retrieved on 2011-11-29.
- ↑ Hunt, Chloe V.; Harvey, James J.; Miller, Anne; Johnson, Vivienne; Phongsuwan, Niphon (2013). "The Green Fins approach for monitoring and promoting environmentally sustainable scuba diving operations in South East Asia". Ocean & Coastal Management 78: 35–44. doi:10.1016/j.ocecoaman.2013.03.004.
- ↑ "Eight great soft corals for new reefkeepers". AquaDaily. 2008-12-05. Retrieved 2009-01-02.
- ↑ Magsaysay, Melissa (June 21, 2009). "Coral makes a splash". Los Angeles Times. Retrieved January 12, 2013.
- ↑ H. Ehrlich, P. Etnoyer, S. D. Litvinov; Etnoyer, P.; Litvinov, S. D.; Olennikova, M.M.; Domaschke, H.; Hanke, T.; Born, R.; Meissner, H.; Worch, H.; et al. (2006). "Biomaterial structure in deep-sea bamboo coral (Anthozoa: Gorgonacea: Isididae)". Materialwissenschaft und Werkstofftechnik (www3.interscience.wiley.com) 37 (6): 552. doi:10.1002/mawe.200600036. Retrieved 2009-05-11.
- ↑ Reddy PN, Lakshmana M, Udupa UV; Lakshmana; Udupa (December 2003). "Effect of Praval bhasma (Coral calx), a natural source of rich calcium on bone mineralization in rats". Pharmacological Research 48 (6): 593–9. doi:10.1016/S1043-6618(03)00224-X. PMID 14527824.
- ↑ Pouwels, Randall L. (6 June 2002). Horn and Crescent: Cultural Change and Traditional Islam on the East African Coast, 800-1900. Cambridge University Press. p. 26. ISBN 978-0-521-52309-7.
- ↑ "Strategic Stone Study: A Building Stone Atlas of Oxfordshire". English Heritage. March 2011. Retrieved 23 April 2015.
- ↑ "National Oceanic and Atmospheric Administration – New Deep-Sea Coral Discovered on NOAA-Supported Mission". www.noaanews.noaa.gov. Retrieved 2009-05-11.
- ↑ Schrag, D.P. and Linsley, B.K. (2002). "Corals, chemistry, and climate". Science 296 (8): 277–278. doi:10.1126/science.1071561. PMID 11951026.
- ↑ Smithers, Scott G.; Woodroffe, Colin D. (2000). "Microatolls as sea-level indicators on a mid-ocean atoll". Marine Geology 168 (1–4): 61–78. doi:10.1016/S0025-3227(00)00043-8.
- ↑ Hoegh-Guldberg O. (1999). "Climate change, coral bleaching and the future of the world’s coral reefs". Marine and Freshwater Research 50: 839–99. doi:10.1071/mf99078.
- 1 2 3 Hughes, T., Baird, A., Bellwood, D., Card, M., Connolly, S., Folke, C., Grosberg, R., Hoegh-Guldberg, O., Jackson, J., Klepas, J., Lough, J., Marshall, P., Nystrom, M., Palumbi, S., Pandolfi, J., Rosen, B., and Roughgarden, J. (2003). "Climate change, human impacts, and the resilience of coral reefs". Science 301: 929–33. doi:10.1126/science.1085046.
- 1 2 Parmesan, C. (2006). "Ecological and evolutionary responses to recent climate change". Annual Review of Ecology, Evolution, and Systematics 37: 637–69. doi:10.1146/annurev.ecolsys.37.091305.110100.
- ↑ Baker, A. (2004). "Corals’ adaptive response to climate change". Nature 430: 741. doi:10.1038/430741a.
- 1 2 Donner, S., Skirving, W., Little, C., Oppenheimer, M., and Hoegh-Guldenberg (2005). "Global assessment of coral bleaching and required rates of adaptation under climate change". Global Change Biology 11: 2251–65. doi:10.1111/j.1365-2486.2005.01073.x.
- ↑ Baskett, M., Gaines, S., and Nisbet, R. (2009). "Symbiont diversity may help coral reefs survive moderate climate change.". Ecological Applications 19: 3–17. doi:10.1890/08-0139.1.
- ↑ McClanahan, T., Ateweberhan, M., Muhando, C., Maina, J., and Mohammed, M. (2007). "Effects of Climate and Seawater Temperature Variation on Coral Bleaching and Morality". Ecological Monographs 77: 503–25. doi:10.1890/06-1182.1.
- ↑ Kilbourne, K. Halimeda; Quinn, Terrence M.; Taylor, Frederick W.; Delcroix, Thierry; Gouriou, Yves (2004). "El Niño-Southern Oscillation-related salinity variations recorded in the skeletal geochemistry of a Porites coral from Espiritu Santo, Vanuatu". Paleoceanography 19 (4): PA4002. doi:10.1029/2004PA001033.
- 1 2 Ren, Lei; Linsley, Braddock K.; Wellington, Gerard M.; Schrag, Daniel P.; Hoegh-guldberg, Ove (2003). "Deconvolving the δ18O seawater component from subseasonal coral δ18O and Sr/Ca at Rarotonga in the southwestern subtropical Pacific for the period 1726 to 1997". Geochimica et Cosmochimica Acta 67 (9): 1609–1621. doi:10.1016/S0016-7037(02)00917-1.
- ↑ Wu, Henry C.; Linsley, Braddock K.; Dassié, Emilie P.; Schiraldi, Benedetto; deMenocal, Peter B. (2013). "Oceanographic variability in the South Pacific Convergence Zone region over the last 210 years from multi-site coral Sr/Ca records". Geochemistry, Geophysics, Geosystems 14 (5): 1435–1453. doi:10.1029/2012GC004293.
- ↑ Kiladis, George N.; von Storch, Hans; van Loon, Harry (1989). "Origin of the South Pacific Convergence Zone". Journal of Climate 2 (10): 1185–1195. doi:10.1175/1520-0442(1989)002<1185:OOTSPC>2.0.CO;2.
- 1 2 Lukas, Roger; Lindstrom, Eric (1991). "The mixed layer of the western equatorial Pacific Ocean". Journal of Geophysical Research 96 (S1): 3343–3358. doi:10.1029/90JC01951.
- ↑ Aquarium Corals: Collection and Aquarium Husbandry of Northeast Pacific Non-Photosynthetic Cnidaria
- ↑ Reefkeeping 101 - Various Nutrient Control Methods
- ↑ Aquarium Substrate & Live Rock Clean Up Tips
- ↑ Excellent Beginner Corals for a Reef Aquarium
- ↑ Coral Reefs
- ↑ Horoszowski-Fridman YB, Izhaki I, Rinkevich B; Izhaki; Rinkevich (2011). "Engineering of coral reef larval supply through transplantation of nursery-farmed gravid colonies". Journal of Experimental Marine Biology and Ecology 399 (2): 162–166. doi:10.1016/j.jembe.2011.01.005.
- ↑ Pomeroy, Robert S.; Parks, John E.; Balboa, Cristina M. (2006). "Farming the reef: Is aquaculture a solution for reducing fishing pressure on coral reefs?". Marine Policy 30 (2): 111–30. doi:10.1016/j.marpol.2004.09.001.
- ↑ Rinkevich B (2008). "Management of coral reefs: We have gone wrong when neglecting active reef restoration" (PDF). Marine pollution bulletin 56 (11): 1821–1824. doi:10.1016/j.marpolbul.2008.08.014. PMID 18829052.
- ↑ Ferse, Sebastian C.A. (2010). "Poor Performance of Corals Transplanted onto Substrates of Short Durability". Restoration Ecology 18 (4): 399–407. doi:10.1111/j.1526-100X.2010.00682.x.
Sources
- Allen, G.R & R. Steene (1994). Indo-Pacific Coral Reef Field Guide. ISBN 981-00-5687-7.
- Calfo, Anthony. Book of Coral Propagation. ISBN 0-9802365-0-9.
- Colin, P.L. & C. Arneson (1995). Tropical Pacific Invertebrates. ISBN 0-9645625-0-2.
- Fagerstrom, J.A. (1987). The Evolution of Reef Communities. ISBN 0-471-81528-4.
- Gosliner, T., D. Behrens & G. Williams (1996). Coral Reef Animals of the Indo-Pacific, Animals Life from Africa to Hawai'i (invertebrates). ISBN 0-930118-21-9.
- Nybakken, J.W. (2004). Marine Biology, An Ecological Approach. ISBN 0-8053-4582-5.
- Redhill, Surrey. Corals of the World: Biology and Field Guide.
- Segaloff, Nat, and Paul Erickson (1991). A Reef Comes to Life. Creating an Undersea Exhibit. ISBN 0-531-10994-1.
- Sheppard, Charles R.C.; Davy, Simon K.; Pilling, Graham M. (25 June 2009). The Biology of Coral Reefs. OUP Oxford. ISBN 978-0-19-105734-2.
- Veron, J.E.N. (1993). Corals of Australia and the Indo-Pacific. ISBN 0-8248-1504-1.
- Wells, Susan. Coral Reefs of the World.
External links
| Wikispecies has information related to: Coral |
| Wikimedia Commons has media related to Coral, |
- "Coral Factsheet". Waitt Institute. Retrieved 2015-06-08.
- What is a coral?
- NOAA CoRIS – Coral Reef Biology
- NOAA Ocean Service Education – Corals
- coral reef identification guide for the Red Sea and Indo-Pacific
- SeaWorld – Coral reef bibliography
- Coral Reefs The Ocean Portal by Smithsonian Institution
- Coral, The Reef & Marine Aquarium Magazine Coral Magazine


