Copepod
| Copepod Temporal range: Early Cretaceous – Recent | |
|---|---|
 | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Crustacea |
| Class: | Maxillopoda |
| Subclass: | Copepoda H. Milne-Edwards, 1840 |
| Orders | |
Copepods (/ˈkoʊpɪpɒd/; meaning "oar-feet") are a group of small crustaceans found in the sea and nearly every freshwater habitat. Some species are planktonic (drifting in sea waters), some are benthic (living on the ocean floor), and some continental species may live in limnoterrestrial habitats and other wet terrestrial places, such as swamps, under leaf fall in wet forests, bogs, springs, ephemeral ponds, and puddles, damp moss, or water-filled recesses (phytotelmata) of plants such as bromeliads and pitcher plants. Many live underground in marine and freshwater caves, sinkholes, or stream beds. Copepods are sometimes used as biodiversity indicators.
Classification and diversity

Copepods form a subclass belonging to the subphylum Crustacea (crustaceans); they are divided into ten orders. Some 13,000 species of copepods are known, and 2,800 of them live in fresh water.[1]
Characteristics
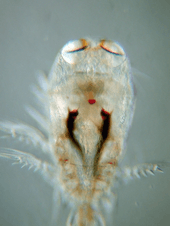
Copepods vary considerably, but can typically be 1 to 2 mm (0.04 to 0.08 in) long, with a teardrop-shaped body and large antennae. Although like other crustaceans, they have an armoured exoskeleton, they are so small that in most species, this thin armour, and the entire body, is almost totally transparent. Some polar copepods reach 1 cm (0.39 in). Most copepods have a single median compound eye, usually bright red and in the centre of the transparent head; subterranean species may be eyeless. Like other crustaceans, copepods possess two pairs of antennae; the first pair is often long and conspicuous.
Copepods typically have a short, cylindrical body, with a rounded or beaked head. The head is fused with the first one or two thoracic segments, while the remainder of the thorax has three to five segments, each with limbs. The first pair of thoracic appendages is modified to form maxillipeds, which assist in feeding. The abdomen is typically narrower than the thorax, and contains five segments without any appendages, except for some tail-like "rami" at the tip.[4]
Because of their small size, copepods have no need of any heart or circulatory system (the members of the order Calanoida have a heart, but no blood vessels), and most also lack gills. Instead, they absorb oxygen directly into their bodies. Their excretory system consists of maxillary glands.
Behavior
The second pair of cephalic appendages in free-living copepods is usually the main time-averaged source of propulsion, beating like oars to pull the animal through the water. However, different groups have different modes of feeding and locomotion, ranging from almost immotile for several minutes (e.g. some harpacticoid copepods) to intermittent motion (e.g., some cyclopoid copepods) and continuous displacements with some escape reactions (e.g. most calanoid copepods.)

Some copepods have extremely fast escape responses when a predator is sensed and can jump with high speed over a few millimetres. Many species have neurons surrounded by myelin (for increased conduction speed), which is very rare among invertebrates (other examples are some annelids and malacostracan crustaceans like palaemonid shrimp and penaeids). Even rarer, the myelin is highly organized, resembling the well-organized wrapping found in vertebrates (Gnathostomata). Despite their fast escape response, copepods are successfully hunted by slow-swimming seahorses, which approach their prey so gradually, it senses no turbulence, then suck the copepod into their snout too suddenly for the copepod to escape.[5]
Finding a mate in the three-dimensional space of open water is challenging. Some copepod females solve the problem by emitting pheromones, which leave a trail in the water that the male can follow.[6]
Diet
copepods feed directly on phytoplankton, catching cells singly. Some of the larger species are predators of their smaller relatives. Many benthic copepods eat organic detritus or the bacteria that grow in it, and their mouth parts are adapted for scraping and biting. Herbivorous copepods, particularly those in rich, cold seas, store up energy from their food as oil droplets while they feed in the spring and summer plankton blooms. These droplets may take up over half of the volume of their bodies in polar species. Many copepods (e.g., fish lice like the Siphonostomatoida) are parasites, and feed on their host organisms.
Diversity in copepod diets is important, as it provides many pathways by which they can obtain their required nutrients. Diets that are nutritionally complete enrich the chances of success amongst individual copepods or the entire copepod population.[7] Copepods are sensitive to metals such as silver, zinc, copper, and nickel.[8] Adding such metals to the diet of copepods leads to a reduction in their feeding and reproduction rates. Of such metals, copepods are the most sensitive to silver,[8] likely because silver is not considered an essential nutrient to copepods or other marine organisms.[9] Copepods generally tend to feed on a mixed algal diet in their natural habitat. The survival success of the copepod population over the years may be partially due to their ability to select prey, avoiding contaminated food.[8]
Life cycle

Copepods are a holoplankton species, meaning they stay planktonic for all of their lifecycle. During mating, the male copepod grips the female with his first pair of antennae, which is sometimes modified for this purpose. The male then produces an adhesive package of sperm and transfers it to the female's genital opening with his thoracic limbs. Eggs are sometimes laid directly into the water, but many species enclose them within a sac attached to the female's body until they hatch. In some pond-dwelling species, the eggs have a tough shell and can lie dormant for extended periods if the pond dries up.[4]
Eggs hatch into nauplius larvae, which consist of a head with a small tail, but no thorax or true abdomen. The nauplius moults five or six times, before emerging as a "copepodid larva". This stage resembles the adult, but has a simple, unsegmented abdomen and only three pairs of thoracic limbs. After a further five moults, the copepod takes on the adult form. The entire process from hatching to adulthood can take a week to a year, depending on the species.[4]
Ecology

Planktonic copepods are important to global ecology and the carbon cycle. They are usually the dominant members of the zooplankton, and are major food organisms for small fish such as the dragonet, banded killifish, whales, seabirds, alaska pollock and other crustaceans such as krill in the ocean and in fresh water. Some scientists say they form the largest animal biomass on earth.[11] Copepods compete for this title with Antarctic krill (Euphausia superba). C. glacialis inhabits the edge of the Arctic icepack, where they alone comprise up to 80% of zooplankton biomass. They bloom as the ice recedes each spring. The ongoing large reductions in the annual minimum of recent years may force them to compete in the open ocean with the much-less nourishing C. finmarchicus, which is spreading from the North Sea and the Norwegian Sea into the Barents Sea.[12]
Because of their smaller size and relatively faster growth rates, and because they are more evenly distributed throughout more of the world's oceans, copepods almost certainly contribute far more to the secondary productivity of the world's oceans, and to the global ocean carbon sink than krill, and perhaps more than all other groups of organisms together. The surface layers of the oceans are currently believed to be the world's largest carbon sink, absorbing about 2 billion tons of carbon a year, the equivalent to perhaps a third of human carbon emissions, thus reducing their impact. Many planktonic copepods feed near the surface at night, then sink (by changing oils into more dense fats)[13][14] into deeper water during the day to avoid visual predators. Their moulted exoskeletons, faecal pellets, and respiration at depth all bring carbon to the deep sea.
About half of the estimated 13,000 described species of copepods are parasitic[15][16] and have strongly modified bodies. They attach themselves to bony fish, sharks, marine mammals, and many kinds of invertebrates such as molluscs, tunicates, or corals. They live as endo- or ectoparasites on fish or invertebrates in fresh water as well as in marine environments.
Copepods as parasitic hosts
In addition to being parasites themselves, copepods are subject to parasitic infection. The most common parasite is the marine dinoflagellate, Blastodinium spp., which are gut parasites of many copepod species.[17][18] Currently 12 species of Blastodinium are described, the majority of which were discovered in the Mediterranean Sea.[17] Most Blastodinium species infect several different hosts, but species-specific infection of copepods does occur. Generally, adult copepod females and juveniles are infected.
During the naupliar stage, the copepod host ingests the unicellular dinospore of the parasite. The dinospore is not digested and continues to grow inside the intestinal lumen of the copepod. Eventually, the parasite divides into a multicellular arrangement called a trophont.[19] This trophont is considered parasitic, contains thousands of cells, and can be several hundred micrometers in length.[18] The trophont is greenish to brownish in color as a result of well-defined chloroplasts. At maturity, the trophont ruptures and Blastodinium spp. are released from the copepod anus as free dinospore cells. Not much is known about the dinospore stage of Blastodinium and its ability to persist outside of the copepod host in relatively high abundances.[20]
The copepod, Calanus finmarchicus, which dominates the northeastern Atlantic coast, has been shown to be greatly infected by this parasite. A 2014 study in this region found up to 58% of collected C. finmarchicus females to be infected.[19] In this study, Blastodinium-infected females had no measurable feeding rate over a 24-hour period. This is compared to uninfected females which, on average, ate 2.93 × 104 cells copepod−1 d−1.[19] Blastodinium-infected females of C. finmarchicus exhibited characteristic signs of starvation including decreased respiration, fecundity, and fecal pellet production. Though photosynthetic, Blastodinium spp. procure most of their energy from organic material in the copepod gut, thus attributing to host starvation.[18] Underdeveloped or disintegrated ovaries, as well as decreased fecal pellet size are a direct result of starvation in female copepods.[21] Infection from Blastodinium spp. could have serious ramifications on the success of copepod species and the function of entire marine ecosystems. Parasitism via Blastodinium spp.' is not lethal, but has negative impacts on copepod physiology, which in turn may alter marine biogeochemical cycles.
Copepods also host Dracunculus medinensis, the Guinea worm nematode that causes dracunculiasis disease in humans. This disease may be close to being eradicated through efforts at the U.S. Centers for Disease Control and Prevention) and the World Health Organization.[22]
Practical aspects
Copepods in marine aquaria
Live copepods are used in the saltwater aquarium hobby as a food source and are generally considered beneficial in most reef tanks. They are scavengers and also may feed on algae, including coralline algae. Live copepods are popular among hobbyists who are attempting to keep particularly difficult species such as the mandarin dragonet or scooter blenny. They are also popular to hobbyists who want to breed marine species in captivity. In a saltwater aquarium, copepods are typically stocked in the refugium.
Water supplies
Copepods are sometimes found in public main water supplies, especially systems where the water is not filtered,[23] such as New York City, Boston, and San Francisco.[24] This is not usually a problem in treated water supplies. In some tropical countries, such as Peru and Bangladesh, a correlation has been found between copepods presence and cholera in untreated water, because the cholera bacteria attach to the surfaces of planktonic animals. The larvae of the guinea worm must develop within a copepod's digestive tract before being transmitted to humans. The risk of infection with these diseases can be reduced by filtering out the copepods (and other matter), for example with a cloth filter.
Copepods have been used successfully in Vietnam to control disease-bearing mosquitoes such as Aedes aegypti that transmit dengue fever and other human parasitic diseases.[25][26]
The copepods can be added to water-storage containers where the mosquitoes breed.[23] Copepods, primarily of the genera Mesocyclops and Macrocyclops (such as Macrocyclops albidus), can survive for periods of months in the containers, if the containers are not completely drained by their users. They attack, kill, and eat the younger first- and second-instar larvae of the mosquitoes. This biological control method is complemented by community trash removal and recycling to eliminate other possible mosquito-breeding sites. Because the water in these containers is drawn from uncontaminated sources such as rainfall, the risk of contamination by cholera bacteria is small, and in fact no cases of cholera have been linked to copepods introduced into water-storage containers. Trials using copepods to control container-breeding mosquitoes are underway in several other countries, including Thailand and the southern United States. The method, though, would be very ill-advised in areas where the guinea worm is endemic.
The matter of copepods in the water supply, however, has raised a problem for some Jewish people who observe Kashrut in that copepods, being crustaceans, are not kosher, and are not small enough to be ignored as nonfood microscopic organisms (since some specimens can be seen with the naked eye). The discovery of copepods in the New York water supply in the summer of 2004 in particular caused significant debate in rabbinical circles and caused some observant Jews to buy filters for their water.[27]
In popular culture
The series Spongebob Squarepants features Plankton who is a copepod.
See also
References
- ↑ Geoff A. Boxhall, Danielle Defaye (2008). E. V. Balian, C. Lévêque, H. Segers & K. Martens, ed. "Freshwater Animal Diversity Assessment". Hydrobiologia 595 (1): 195–207. doi:10.1007/s10750-007-9014-4.
|chapter=ignored (help) - ↑ Ivan R. Schwab (2012). Evolution's Witness: How Eyes Evolved. Oxford University Press. p. 231. ISBN 9780195369748.
- ↑ Charles B. Miller (2004). Biological Oceanography. John Wiley & Sons. p. 122. ISBN 9780632055364.
- 1 2 3 Robert D. Barnes (1982). Invertebrate Zoology. Philadelphia, Pennsylvania: Holt-Saunders International. pp. 683–692. ISBN 0-03-056747-5.
- ↑ "Seahorses stalk their prey by stealth". BBC News. November 26, 2013.
- ↑ David B. Dusenbery (2009). Living at Micro Scale. Cambridge, Massachusetts: Harvard University Press. p. 306. ISBN 978-0-674-03116-6.
- ↑ G. S. Kleppel (1993). "On the diets of calanoid copepods" (PDF). Marine Ecology Progress Series 99: 183–183. doi:10.3354/meps099183.
- 1 2 3 Gretchen K. Bielmyer, Martin Grosell & Kevin V. Brix (2006). "Toxicity of silver, zinc, copper, and nickel to the copepod Acartia tonsa exposed via a phytoplankton diet" (PDF). Environmental Science & Technology 40 (6): 2063–2068. doi:10.1021/es051589a. PMID 16570637.
- ↑ Mariana Saia Pedroso, José Guilherme Filho Bersano & Adalto Bianchini (2007). "Acute silver toxicity in the euryhaline copepod Acartia tonsa: influence of salinity and food". Environmental Toxicology and Chemistry 26 (10): 2158–2165. doi:10.1897/06-485R.1. PMID 17867869.
- ↑ Justine, JL.; Beveridge, I.; Boxshall, GA.; Bray, RA.; Miller, TL.; Moravec, F.; Trilles, JP.; Whittington, ID. (2012). "An annotated list of fish parasites (Isopoda, Copepoda, Monogenea, Digenea, Cestoda, Nematoda) collected from Snappers and Bream (Lutjanidae, Nemipteridae, Caesionidae) in New Caledonia confirms high parasite biodiversity on coral reef fish.". Aquat Biosyst 8 (1): 22. doi:10.1186/2046-9063-8-22. PMC 3507714. PMID 22947621.
- ↑ Johannes Dürbaum & Thorsten Künnemann (November 5, 1997). "Biology of Copepods: An Introduction". Carl von Ossietzky University of Oldenburg. Retrieved December 8, 2009.
- ↑ "Biodiversity: Pity the copepod". The Economist. June 16, 2012. pp. 8–9. Retrieved 2012-06-19.
- ↑ David W. Pond & Geraint A. Tarling (2011). "Phase transitions of wax esters adjust buoyancy in diapausing Calanoides acutus". Limnology and Oceanography 56 (4): 1310–1318. doi:10.4319/lo.2011.56.4.1310.
- ↑ David W. Pond & Geraint A. Tarling (13 June 2011). "Copepods share "diver's weight belt" technique with whales". British Antarctic Survey. Retrieved November 20, 2012.
- ↑ H. L. Suh, J. D. Shim and S. D. Choi (1992). "Four Species of Copepoda (Poecilostomatoida) Parasitic on Marine Fishes of Korea". Bulletin of the Korean Fisheries Society 25 (4): 291–300. (in Korean with English abstract)
- ↑ See photograph at "Blobfish / Psychrolutes microporos" (PDF). Census of Marine Life / NIWA. Retrieved December 9, 2007. Photograph taken by Kerryn Parkinson and Robin McPhee in June 2003.
- 1 2 Edouard Chatton (1920). "Les Pe´ridiniens parasites. Morphologie, reproduction, e´thologie.". http://www.im.microbios.org/0903/0903173.pdf. Arch. Zool. Exp. Ge´n.,. pp. 59, 1–475. plates I–XVIII. External link in
|website=(help); - 1 2 3 Skovgaard, Alf; Karpov, Sergey A.; Guillou, Laure (2012). "The Parasitic Dinoflagellates Blastodinium spp. Inhabiting the Gut of Marine, Planktonic Copepods: Morphology, Ecology, and Unrecognized Species Diversity". Front Microbiol. 3:305. doi:10.3389/fmicb.2012.00305.
- 1 2 3 Fields, D.M.; Runge, J.A.; Thompson, C.; Shema, S.D.; Bjelland, R.M.; Durif, C.M.F.; Skiftesvik, A.B.; Browman, H.I. (2014). "Infection of the planktonic copepod Calanus finmarchicus by the parasitic dinoflagellate, Blastodinium spp: effects on grazing, respiration, fecundity and fecal pellet production". J. Plankton Res. 0(0): 1–10. doi:10.1093/plankt/fbu084.
- ↑ Alves-de-Souza, Catharina; Cornet, C; Nowaczyk, A; Gasparini, Stéphane; Skovgaard, Alf; Guillou, Laure (2011). "Blastodinium spp. infect copepods in the ultra-oligotrophic marine waters of the Mediterranean Sea". Biogeosciences 8: 2125–2136. doi:10.5194/bgd-8-2563-2011.
- ↑ Niehoff, Barbara (2000). "Effect of starvation on the reproductive potential of Calanus finmarchicus". ces Journal of Marine Science. 57(6): 1764–1772. doi:10.1006/jmsc.2000.0971.
- ↑ "This Species is Close to Extinction and That's a Good Thing". Time. January 23, 2015.
- 1 2 Drink Up NYC: Meet The Tiny Crustaceans (Not Kosher) In Your Tap Water. Time, Sept. 2010, Allie Townsend.
- ↑ Anthony DePalma (July 20, 2006). "New York's water supply may need filtering". The New York Times. Retrieved October 12, 2010.
- ↑ Vu Sinh Nam, Nguyen Thi Yen, Tran Vu Pong, Truong Uyen Ninh, Le Quyen Mai, Le Viet, Lo, Le Trung Nghia, Ahmet Bektas, Alistair Briscombe, John G. Aaskov, Peter A. Ryan & Brian H. Kay (1 January 2005). "Elimination of dengue by community programs using Mesocyclops (Copepoda) against Aedes aegypti in central Vietnam". American Journal of Tropical Medicine and Hygiene 72 (1): 67–73. PMID 15728869.
- ↑ G. G. Marten & J. W. Reid (2007). "Cyclopoid copepods". Journal of the American Mosquito Control Association 23 (2 Suppl.): 65–92. doi:10.2987/8756-971X(2007)23[65:CC]2.0.CO;2. PMID 17853599.
- ↑ "OU Fact Sheet on NYC Water". Orthodox Union Kosher Certification. New York City: Orthodox Union. August 13, 2004. Retrieved May 1, 2013. External link in
|work=(help)
External links
| Wikimedia Commons has media related to Copepoda. |
| Wikispecies has information related to: Copepoda |
- Copepod fact sheet - Guide to the marine zooplankton of south eastern Australia
- Diversity and geographical distribution of pelagic copepoda
- Copepod World
- Neotropical Copepoda Database Project
- The World Copepod Culture Database
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|