Thalassemia
| Thalassemia | |
|---|---|
| Classification and external resources | |
| Specialty | Hematology |
| ICD-10 | D56 |
| ICD-9-CM | 282.4 |
| OMIM | 141800 141850 142310 604131 141800 141850 142310 604131 |
| DiseasesDB | 448 33334 |
| MedlinePlus | 000587 |
| eMedicine | article/958850 article/206490 article/955496 article/396792 |
| Patient UK | Thalassemia |
| MeSH | D013789 |
| GeneReviews | |
Thalassemia (British English: thalassaemia), also called Mediterranean anemia, is a form of inherited autosomal recessive blood disorder characterized by abnormal formation of hemoglobin.[1] The abnormal hemoglobin formed results in improper oxygen transport and destruction of red blood cells.[1] Thalassemia is caused by variant or missing genes that affect how the body makes hemoglobin, the protein in red blood cells that carries oxygen. People with thalassemia make less hemoglobin and have fewer circulating red blood cells than normal, which results in mild or severe microcytic anemia.
Thalassemia can cause complications, including iron overload, bone deformities, and cardiovascular illness. However, this same inherited disease of red blood cells may confer a degree of protection against malaria (specifically, malaria caused by the protozoan parasite Plasmodium falciparum), which is or was prevalent in the regions where the trait is common. This selective survival advantage of carriers (known as heterozygous advantage) may be responsible for perpetuating the mutation in populations. In that respect, the various thalassemias resemble another genetic disorder affecting hemoglobin, sickle-cell disease.[2][3]
Thalassemia resulted in 25,000 deaths in 2013 down from 36,000 deaths in 1990.[4]
Signs and symptoms
- Iron overload: People with thalassemia can get an overload of iron in their bodies, either from the disease itself or from frequent blood transfusions. Too much iron can result in damage to the heart, liver, and endocrine system, which includes glands that produce hormones that regulate processes throughout the body. The damage is characterized by excessive deposits of iron. Without adequate iron chelation therapy, almost all patients with beta-thalassemia accumulate potentially fatal iron levels.[5]
- Infection: People with thalassemia have an increased risk of infection. This is especially true if the spleen has been removed.
- Bone deformities: Thalassemia can make the bone marrow expand, which causes bones to widen. This can result in abnormal bone structure, especially in the face and skull. Bone marrow expansion also makes bones thin and brittle, increasing the risk of broken bones.[6]
- Enlarged spleen: The spleen aids in fighting infection and filters unwanted material, such as old or damaged blood cells. Thalassemia is often accompanied by the destruction of a large number of red blood cells and the task of removing these cells causes the spleen to enlarge. Splenomegaly can make anemia worse, and it can reduce the life of transfused red blood cells. Severe enlargement of the spleen may necessitate its removal.
- Slowed growth rates: Anemia can cause a child's growth to slow. Puberty also may be delayed in children with thalassemia.
- Heart problems: Diseases, such as congestive heart failure and abnormal heart rhythms, may be associated with severe thalassemia.[7]
Cause
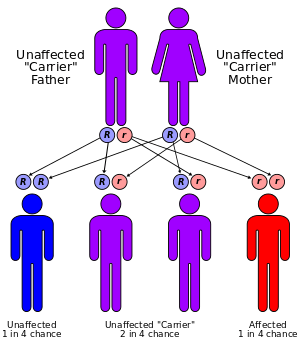
Both α- and β-thalassemias are often inherited in an autosomal recessive manner. Cases of dominantly inherited α- and β-thalassemias have been reported, the first of which was in an Irish family with two deletions of 4 and 11 bp in exon 3 interrupted by an insertion of 5 bp in the β-globin gene. For the autosomal recessive forms of the disease, both parents must be carriers for a child to be affected. If both parents carry a hemoglobinopathy trait, the risk is 25% for each pregnancy for an affected child. Genetic counseling and genetic testing are recommended for families who carry a thalassemia trait.
An estimated 60-80 million people in the world carry the β-thalassemia trait. This is a rough estimate; the actual number of those with thalassemia major is unknown due to the prevalence of thalassemia in less-developed countries. Countries such as Nepal, Bangladesh and Pakistan are seeing a large increase of thalassemia patients due to lack of genetic counseling and screening. Concern is increasing that thalassemia may become a very serious problem in the next 50 years, one that will burden the world's blood bank supplies and the health system in general. An estimated 1,000 people live with thalassemia major in the United States, and an unknown number of carriers. Because of the prevalence of the disease in countries with little knowledge of thalassemia, access to proper treatment and diagnosis can be difficult.
Evolution
Having a single gene for thalassemia may protect against malaria and thus be an advantage.[8]
People diagnosed with heterozygous (carrier) β-thalassemia have some protection against coronary heart disease.[9]
Pathophysiology
Normally, the majority of adult hemoglobin (HbA) is composed of four protein chains, two α and two β globin chains arranged into a heterotetramer. In thalassemia, patients have defects in either the α or β globin chain, causing production of abnormal red blood cells (In sickle-cell disease, the mutation is specific to β globin).
The thalassemias are classified according to which chain of the hemoglobin molecule is affected. In α-thalassemias, production of the α globin chain is affected, while in β-thalassemia, production of the β globin chain is affected.
The β globin chains are encoded by a single gene on chromosome 11; α globin chains are encoded by two closely linked genes on chromosome 16.[10] Thus, in a normal person with two copies of each chromosome, two loci encode the β chain, and four loci encode the α chain. Deletion of one of the α loci has a high prevalence in people of African or Asian descent, making them more likely to develop α-thalassemia. β-Thalassemias are not only common in Africans, but also in Greeks and Italians.
Alpha-thalassemias
The α-thalassemias involve the genes HBA1[11] and HBA2,[12] inherited in a Mendelian recessive fashion. Two gene loci and so four alleles exist. It is also connected to the deletion of the 16p chromosome. α Thalassemias result in decreased alpha-globin production, therefore fewer alpha-globin chains are produced, resulting in an excess of β chains in adults and excess γ chains in newborns. The excess β chains form unstable tetramers (called hemoglobin H or HbH of 4 beta chains), which have abnormal oxygen dissociation curves.
Beta-thalassemia
Beta thalassemias are due to mutations in the HBB gene on chromosome 11,[13] also inherited in an autosomal, recessive fashion. The severity of the disease depends on the nature of the mutation. Mutations are characterized as either βo or β thalassemia major if they prevent any formation of β chains, the most severe form of β-thalassemia; as either β+ or β thalassemia intermedia if they allow some β chain formation to occur; or as β thalassemia minor if only one of the two β globin alleles contains a mutation, so that β chain production is not terribly compromised and patients may be relatively asymptomatic.
Delta-thalassemia
As well as alpha and beta chains present in hemoglobin, about 3% of adult hemoglobin is made of alpha and delta chains. Just as with beta thalassemia, mutations that affect the ability of this gene to produce delta chains can occur.
Combination hemoglobinopathies
Thalassemia can coexist with other hemoglobinopathies. The most common of these are:
- Hemoglobin E/thalassemia: common in Cambodia, Thailand, and parts of India, it is clinically similar to β thalassemia major or thalassemia intermedia.
- Hemoglobin S/thalassemia: common in African and Mediterranean populations, is clinically similar to sickle-cell anemia, with the additional feature of splenomegaly.
- Hemoglobin C/thalassemia: common in Mediterranean and African populations, hemoglobin C/βo thalassemia causes a moderately severe hemolytic anemia with splenomegaly; hemoglobin C/β+ thalassemia produces a milder disease.
- Hemoglobin D/thalassemia: common in the northwestern parts of India and Pakistan (Punjab region).
Management
Mild thalassemia: people with thalassemia traits do not require medical or follow-up care after the initial diagnosis is made.[14] People with β-thalassemia trait should be warned that their condition can be misdiagnosed as the more common iron deficiency anemia. They should avoid routine use of iron supplements; iron deficiency can develop, though, during pregnancy or from chronic bleeding.[15] Counseling is indicated in all persons with genetic disorders, especially when the family is at risk of a severe form of disease that may be prevented.[16]
Severe thalassemia: People with severe thalassemia require medical treatment. A blood transfusion regimen was the first measure effective in prolonging life.[14]
Medications
Multiple blood transfusions can result in iron overload. The iron overload related to thalassemia may be treated by chelation therapy with the medications deferoxamine, deferiprone, or deferasirox.[17] These treatments have resulted in improved life expectancy in those with thalassemia major.[17]
Deferoxamine is only effective via daily injections which makes its long-term use more difficult. It has the benefit of being inexpensive and decent long-term safety. Adverse effects are primary skin reactions around the injection site and hearing loss.[17]
Deferasirox has the benefit of being an oral medication. Common side effects include: nausea, vomiting and diarrhea. It however is not effective in everyone and is probably not suitable in those with significant cardiac issues related to iron overload. The cost is also significant.[17]
Deferiprone is given as an oral medication. Nausea, vomiting, and diarrhea are relatively common with its use. While available in Europe as of 2010, it is not available in North America. It appears to be the most effective agent when the heart is significantly involved.[17]
There is no evidence from randomised controlled trial to support zinc supplementation in thalassemia.[18]
Carrier detection
- A screening policy exists in Cyprus to reduce the incidence of thalassemia, which, since the program's implementation in the 1970s (which also includes prenatal screening and abortion), has reduced the number of children born with the hereditary blood disease from one of every 158 births to almost zero.[19]
- In Iran as a premarital screening, the man's red cell indices are checked first, if he has microcytosis (mean cell hemoglobin < 27 pg or mean red cell volume < 80 fl), the woman is tested. When both are microcytic, their hemoglobin A2 concentrations are measured. If both have a concentration above 3.5% (diagnostic of thalassemia trait) they are referred to the local designated health post for genetic counseling.[20]
- Large scale awareness campaigns are being organized in India both by government and non-government organizations in favor of voluntary premarital screening to detect carriers of thalassemia and marriage between both carriers are strongly discouraged.
Bone marrow transplant
Bone marrow transplantation may offer the possibility of a cure in young people who have an HLA-matched donor.[21] Success rates have been in the 80–90% range.[21] Mortality from the procedure is about 3%.[22] There are no randomized controlled trials which have tested the safety and efficacy of non-identical donor bone marrow transplantation in persons with β- thallassemia who are dependent on blood transfusion.[23]
If the person does not have an HLA-matched compatible donor, another method called bone marrow transplantation (BMT) from haploidentical mother to child (mismatched donor) may be used. In a study of 31 people, the thalassemia-free survival rate 70%, rejection 23%, and mortality 7%. The best results are with very young people.[24]
Epidemiology
The beta form of thalassemia is particularly prevalent among Mediterranean peoples, and this geographical association is responsible for its naming.[25] Thalassemia resulted in 25,000 deaths in 2013 down from 36,000 deaths in 1990.[4]
In Europe, the highest concentrations of the disease are found in Greece, coastal regions in Turkey (particularly the Aegean Region such as Izmir, Balikesir, Aydin, Mugla, and Mediterranean Region such as Antalya, Adana, Mersin), in parts of Italy, particularly southern Italy and the lower Po valley. The major Mediterranean islands (except the Balearics) such as Sicily, Sardinia, Malta, Corsica, Cyprus, and Crete are heavily affected in particular. Other Mediterranean people, as well as those in the vicinity of the Mediterranean, also have high rates of thalassemia, including people from West Asia and North Africa. Far from the Mediterranean, South Asians are also affected, with the world's highest concentration of carriers (16% of the population) being in the Maldives.
Nowadays, it is found in populations living in Africa, the Americas, and in Tharu people in the Terai region of Nepal and India.[26] It is believed to account for much lower malaria sicknesses and deaths,[27] accounting for the historic ability of Tharus to survive in areas with heavy malaria infestation, where others could not. Thalassemias are particularly associated with people of Mediterranean origin, Arabs (especially Palestinians and people of Palestinian descent), and Asians.[28] The Maldives has the highest incidence of Thalassemia in the world with a carrier rate of 18% of the population. The estimated prevalence is 16% in people from Cyprus, 1%[29] in Thailand, and 3–8% in populations from Bangladesh, China, India, Malaysia and Pakistan. Thalassemias also occur in descendants of people from Latin America and Mediterranean countries (e.g. Greece, Italy, Portugal, Spain, and others).
Etymology
The name of this condition derives from the Greek thalassa (θάλασσα), sea, and haema (αἷμα), blood. The term was first used in 1932.[25]:877[30]
Society and culture
In 2008, in Spain, a baby was selectively implanted to be a cure for his brother's thalassemia. The child was born from an embryo screened to be free of the disease before implantation with in vitro fertilization. The baby's supply of immunologically compatible cord blood was saved for transplantation to his brother. The transplantation was considered successful.[31] In 2009, a group of doctors and specialists in Chennai and Coimbatore registered the successful treatment of thalassemia in a child using an unaffected sibling's umbilical cord blood.[32]
References
- 1 2 Mayo Clinic. "Thalassemia". Mayo Clinic. Retrieved 17 October 2014.
- ↑ Weatherall, David J. "Ch. 47: The Thalassemias: Disorders of Globin Synthesis". In Lichtman MA, Kipps TJ, Seligsohn U, Kaushansky K, Prchal, JT. Williams Hematology (8e ed.).
- ↑ "Complications". Thalassemia. Mayo Clinic. Feb 4, 2011. Retrieved 20 September 2011.
- 1 2 GBD 2013 Mortality and Causes of Death, Collaborators (17 December 2014). "Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013.". Lancet 385: 117–71. doi:10.1016/S0140-6736(14)61682-2. PMC 4340604. PMID 25530442.
- ↑ Cianciulli P (October 2008). "Treatment of iron overload in thalassemia". Pediatr Endocrinol Rev 6 (Suppl 1): 208–13. PMID 19337180.
- ↑ Vogiatzi, Maria G; Macklin, Eric A; Fung, Ellen B; Cheung, Angela M; Vichinsky, Elliot; Olivieri, Nancy; Kirby, Melanie; Kwiatkowski, Janet L; Cunningham, Melody; Holm, Ingrid A; Lane, Joseph; Schneider, Robert; Fleisher, Martin; Grady, Robert W; Peterson, Charles C; Giardina, Patricia J (March 2009). "Bone Disease in Thalassemia: A Frequent and Still Unresolved Problem". Journal of Bone and Mineral Research 24 (3): 543–557. doi:10.1359/jbmr.080505. ISSN 0884-0431. PMC 3276604. PMID 18505376.
- ↑ "Thalassemia Complications". Thalassemia. Open Publishing. Retrieved 27 September 2011.
- ↑ Wambua S; Mwangi, Tabitha W.; Kortok, Moses; Uyoga, Sophie M.; Macharia, Alex W.; Mwacharo, Jedidah K.; Weatherall, David J.; Snow, Robert W.; Marsh, Kevin; Williams, Thomas N. (May 2006). "The Effect of α +-Thalassaemia on the Incidence of Malaria and Other Diseases in Children Living on the Coast of Kenya". PLoS Medicine 3 (5): e158. doi:10.1371/journal.pmed.0030158. PMC 1435778. PMID 16605300.
- ↑ Tassiopoulos S; Deftereos, Spyros; Konstantopoulos, Kostas; Farmakis, Dimitris; Tsironi, Maria; Kyriakidis, Michalis; Aessopos, Athanassios (2005). "Does heterozygous beta-thalassemia confer a protection against coronary artery disease?". Annals of the New York Academy of Sciences 1054: 467–70. doi:10.1196/annals.1345.068. PMID 16339699.
- ↑ Robbins Basic Pathology, Page No:428
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) Hemoglobin—Alpha locus 1; HBA1 -141800
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) Hemoglobin—Alpha locus 2; HBA2 -141850
- ↑ Online 'Mendelian Inheritance in Man' (OMIM) Hemoglobin—Beta Locus; HBB -141900
- 1 2 Pediatric Thalassemia~treatment at eMedicine
- ↑ Burdick CO; Ntaios, G.; Rathod, D. (March 2009). "Separating thalassemia trait and iron deficiency by simple inspection". Am. J. Clin. Pathol. 131 (3): 444; author reply 445. doi:10.1309/AJCPC09VRAXEASMH. PMID 19228649.
- ↑ Harrison's Principles of Internal Medicine (17th ed.). McGraw-Hill medical. September 2008. p. 776. ISBN 0-07-164114-9.
- 1 2 3 4 5 Neufeld, EJ (2010). "Update on iron chelators in thalassemia.". Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program 2010: 451–5. doi:10.1182/asheducation-2010.1.451. PMID 21239834.
- ↑ Kye Mon Min Swe (2013). "Zinc supplements for treating thalassaemia and sickle cell disease". Cochrane Database of Systematic Reviews (6): Art. No.: CD009415. doi:10.1002/14651858.CD009415.pub2.
- ↑ Leung TN, Lau TK, Chung TKh (April 2005). "Thalassaemia screening in pregnancy". Current Opinion in Obstetrics and Gynecology 17 (2): 129–34. doi:10.1097/01.gco.0000162180.22984.a3. PMID 15758603.
- ↑ Samavat A, Modell B (November 2004). "Iranian national thalassaemia screening programme". BMJ (Clinical Research Ed.) 329 (7475): 1134–7. doi:10.1136/bmj.329.7475.1134. PMC 527686. PMID 15539666.
- 1 2 Gaziev, J; Lucarelli, G (June 2011). "Hematopoietic stem cell transplantation for thalassemia.". Current stem cell research & therapy 6 (2): 162–9. doi:10.2174/157488811795495413. PMID 21190532.
- ↑ Sabloff, M; Chandy, M; Wang, Z; Logan, BR; Ghavamzadeh, A; Li, CK; Irfan, SM; Bredeson, CN; et al. (2011). "HLA-matched sibling bone marrow transplantation for β-thalassemia major". Blood 117 (5): 1745–50. doi:10.1182/blood-2010-09-306829. PMC 3056598. PMID 21119108.
- ↑ Jagannath, Vanitha A (2014). "Hematopoietic stem cell transplantation for people with ß-thalassaemia major". Cochrane Database of Systematic Reviews 10 (10): Art. No.: CD008708. doi:10.1002/14651858.CD008708.pub3. PMID 25316103. Retrieved 18 October 2014.
- ↑ Sodani, P; Isgrò, A; Gaziev, J; Paciaroni, K; Marziali, M; Simone, MD; Roveda, A; De Angelis, G; et al. (2011). "T cell-depleted hla-haploidentical stem cell transplantation in thalassemia young patients". Pediatric reports 3 (Suppl 2): e13. doi:10.4081/pr.2011.s2.e13. PMC 3206538. PMID 22053275.
- 1 2 John P. Greer JP, Arber DA, Glader B, et al. Wintrobe's Clinical Hematology 2013. ISBN 9781451172683
- ↑ Modiano, G.; Morpurgo, G; Terrenato, L; Novelletto, A; Di Rienzo, A; Colombo, B; Purpura, M; Mariani, M; et al. (1991). "Protection against malaria morbidity: Near-fixation of the α-thalassemia gene in a Nepalese population". American Journal of Human Genetics 48 (2): 390–7. PMC 1683029. PMID 1990845.
- ↑ Terrenato, L; Shrestha, S; Dixit, KA; Luzzatto, L; Modiano, G; Morpurgo, G; Arese, P (February 1988). "Decreased malaria morbidity in the Tharu people compared to sympatric populations in Nepal.". Annals of tropical medicine and parasitology 82 (1): 1–11. PMID 3041928.
- ↑ E. Goljan, Pathology, 2nd ed. Mosby Elsevier, Rapid Review Series.
- ↑ "Thalassemia" (in Thai). Department of Medical Sciences,. September 2011. Archived from the original on 2011-09-25.
- ↑ Whipple GH, Bradford WI. Am J Dis Child 1932;44:336
- ↑ Spanish Baby Engineered To Cure Brother
- ↑ His sister's keeper: Brother's blood is boon of life, Times of India, 17 September 2009
External links
- Thalassemia at DMOZ
- Information on Thalassemia
- Learning About Thalassemia published by the National Human Genome Research Institute.
- Cardiff Sickle Cell & Thalassaemia Centre