Constrained geometry complex
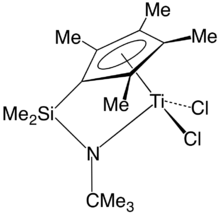 A constrained geometry organotitanium complex in the (inactive) chloride form.
A constrained geometry organotitanium complex in the (inactive) chloride form.
The term "constrained geometry complex" (CGC) (sometimes also referred as Bercaw's Complex) was first used in a patent issued by The Dow Chemical Company for complexes in which a pi-bonded moiety (e.g. cyclopentadienyl) is linked to one of the other ligands on the same metal centre in such a way that the angle at this metal between the centroid of the pi-system and the additional ligand is smaller than in comparable unbridged complexes.[1] More specifically, the term CGC was used for ansa-bridged cyclopentadienyl amido complexes, though the definition goes far beyond this class of compounds. Hence, in the literature the term CGC is frequently used in connection with other more or less related ligand systems that may or may not be isolobal and/or isoelectronic with the ansa-bridged cyclopentadienyl amido ligand system. Furthermore, the term is frequently used for related complexes with long ansa-bridges that induce no strain. Ansa-bridged cyclopentadienyl amido complexes are known for the Group 3, 4, 5, 6 and some Group 8 metals, with the Group 4 congeners being the most studied ones.
Like Group 4 metallocenes, suitable Group 4 CGCs may be activated for the polymerisation of ethylene and alpha-olefins by reaction with co-catalysts, e.g. methylaluminoxane (MAO), perfluorinated boranes and trityl borates. The catalytic systems based on CGCs, however, display incorporation of higher alpha-olefins to a much larger extend than comparable metallocene based systems. This superiority of CGCs in copolymerisation reactions is ascribed to (i) a high accessibility of the reactive centre and (ii) a low tendency of the bulk polymer chain to undergo chain transfer reactions. CGC derived polymers are currently marketed by The Dow Chemical Company as part of their INSITE technology.[2] Polymers based upon INSITE technology began commercial production in the early 1990s[3] and today are made at the billion pound scale.[4][5]
Beyond the use of CGCs for polymerisation reactions, a number of other transformations catalysed by CGCs (both of Group 3 and 4 metals) have been reported from academic laboratories. These include the application of CGCs as catalysts for hydrogenation of imines, hydroboration of alkenes, carboalumination of alkenes, hydrosilylation of alkenes, hydroamination/cyclisation of alpha,omega-aminoalkenes and dimerisation of terminal alkynes.
References
- ↑ Holger Braunschweig and Frank M. Breitling (2006). "Constrained geometry complexes—Synthesis and applications". Coordination Chemistry Reviews 250 (21–22): 2691–2720. doi:10.1016/j.ccr.2005.10.022.
- ↑ Chum, P. S., W. J. Kruper, and M. J. Guest. "Materials properties derived from INSITE metallocene catalysts." Advanced Materials 12.23 (2000): 1759-1767.
- ↑ "Success is Insite for Dow's unique patented catalyst technology", ICIS Chemical Business, 4 January 1997.
- ↑ Lloyd, Lawrie (2011). Handbook of industrial catalysts. Springer. p. 334.
- ↑ Dow Chemical. Solutionism at Work: 2011 Databook, www.dow.com/financial, Form No. 161-00770, April 2012, page 62.