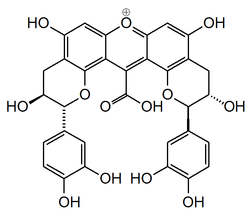
Compound NJ2
 | |
| Identifiers | |
|---|---|
| Jmol interactive 3D | Image |
| |
| Properties | |
| C32H25O13 | |
| Molar mass | 617.53 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Compound NJ2 is a xanthylium yellowish pigment found in wine.
In model solutions, colorless compounds, such as catechin, can give rise to new types of pigments. The first step is the formation of colorless dimeric compounds consisting of two flavanol units linked by carboxy-methine bridge. This is followed by the formation of xanthylium salt yellowish pigments and their ethylesters, resulting from the dehydration of the colorless dimers, followed by an oxidation process. The loss of a water molecule takes place between two A ring hydroxyl groups of the colorless dimers.[1]
See also
References
- ↑ Xanthylium salts formation involved in wine colour changes. Nour-Eddine Es-Safi, Christine Le Guernevé, Hélène Fulcrand, Véronique Cheynier and Michel Moutounet, International Journal of Food Science & Technology, February 2000, Volume 35, Issue 1, pages 63–74, doi:10.1046/j.1365-2621.2000.00339.x
This article is issued from Wikipedia - version of the Tuesday, July 07, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.