Colony-forming unit
In microbiology, a colony-forming unit (CFU) is a unit used to estimate the number of viable bacteria or fungal cells in a sample. Viable is defined as the ability to multiply via binary fission under the controlled conditions. Counting with colony-forming units requires culturing the microbes and counts only viable cells, in contrast with microscopic examination which counts all cells, living or dead. The visual appearance of a colony in a cell culture requires significant growth, and when counting colonies it is uncertain if the colony arose from one cell or a group of cells. Expressing results as colony-forming units reflects this uncertainty.
Theory
The purpose of plate counting is to estimate the number of cells present based on their ability to give rise to colonies under specific conditions of nutrient medium, temperature and time.Theoretically, one viable cell can give rise to a colony through replication. However, solitary cells are the exception in nature, and most likely the progenitor of the colony was a mass of cells deposited together. In addition, many bacteria grow in chains (e.g. Streptococcus) or clumps (e.g. Staphylococcus). Estimation of microbial numbers by CFU will, in most cases, undercount the number of living cells present in a sample for these reasons. This is because the counting of CFU assumes that every colony is separate and founded by a single viable microbial cell.[1]
The plate count is linear for E. coli over the range of 30 - 300 CFU on a standard sized Petri dish.[2] Therefore, to ensure that a sample will yield CFU in this range requires dilution of the sample and plating of several dilutions. Typically ten-fold dilutions are used, and the dilution series is plated in replicates of 2 or 3 over the chosen range of dilutions. The CFU/plate is read from a plate in the linear range, and then the CFU/g (or CFU/mL) of the original is deduced mathematically, factoring in the amount plated and its dilution factor.
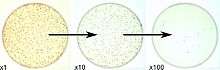
An advantage to this method is that different microbial species may give rise to colonies that are clearly different from each other, both microscopically and macroscopically. The colony morphology can be of great use in the identification of the microorganism present.
A prior understanding of the microscopic anatomy of the organism can give a better understanding of how the observed CFU/mL relates to the number of viable cells per milliliter. Alternatively it is possible to decrease the average number of cells per CFU in some cases by vortexing the sample before conducting the dilution. However many microorganisms are delicate and would suffer a decrease in the proportion of cells that are viable when placed in a vortex.
Uses
Colony-forming units are used to quantify results in many microbiological plating and counting methods, including:
- The Pour Plate method wherein the sample is suspended in a petri dish using molten agar cooled to approximately 40-45 °C (just above the point of solidification to minimize heat-induced cell death). After the nutrient agar solidifies the plate is incubated.[3]
- The Spread Plate method wherein the sample (in a small volume) is spread across the surface of a nutrient agar plate and allowed to dry before incubation for counting.[3]
- The Membrane Filter method wherein the sample is filtered through a membrane filter, then the filter placed on the surface of a nutrient agar plate (bacteria side up). During incubation nutrients leach up through the filter to support the growing cells. As the surface area of most filters is less than that of a standard petri dish, the linear range of the plate count will be less.[3]
- The Miles and Misra Methods or drop-plate method wherein a very small aliquot (usually about 10 microliters) of sample from each dilution in series is dropped onto a petri dish. The drop dish must be read while the colonies are very small to prevent the loss of CFU as they grow together.
However, with the techniques that require the use of an agar plate, no fluid solution can be used because the purity of the specimen cannot be unidentified and it is not possible to count the cells one by one in the liquid.[4]
Tools for counting colonies

Counting colonies is traditionally performed manually using a pen and a click-counter. This is generally a straightforward task, but can become very laborious and time consuming when many plates have to be enumerated. Alternatively semi-automatic (software) and automatic (hardware + software) solutions can be used.
Software for counting CFUs
Colonies can be enumerated from pictures of plates using software tools. The experimenters would generally take a picture of each plate they need to count and then analyse all the pictures (this can be done with a simple digital camera or even a webcam). Since it takes less than 10 seconds to take a single picture, as opposed to several minutes to count CFU manually, this approach generally saves a lot of time. In addition, it is more objective and allows extraction of other variables such as the size and colour of the colonies.
- OpenCFU is a free and open-source program designed to optimise user friendliness, speed and robustness. It offers a wide range of filters and control as well as a modern user interface. OpenCFU is written in C++ and uses OpenCV for image analysis.[5]
- NICE is a program written in MATLAB providing an easy way to count colonies from images.[6][7]
- ImageJ and CellProfiler: Some ImageJ macros[8] and plugins and some CellProfiler pipelines[9] can be used to count colonies. This often requires the user to change the code in order to achieve an efficient work-flow, but can prove useful and flexible. One main issue is the absence of specific GUI which can make the interaction with the processing algorithms tedious.
Automated systems
Many of the automated systems are used to counteract human error as many of the research techniques done by humans counting individual cells have a high chance of error involved. Due to the fact that researchers regularly manually count the cells with the assistance of a transmitted light, this error prone technique can have a significant effect on the calculated concentration in the main liquid medium when the cells are in low numbers.

Completely automated systems are also available from some biotechnology manufacturers. They are generally expensive and not as flexible as standalone software since the hardware and software are designed to work together for a specific set-up. Alternatively, some automatic systems use the spiral plating paradigm.
Some of the automated systems such as the systems from MATLAB allow the cells to be counted without having to stain them. This lets the colonies to be reused for other experiments without the risk of killing the microorganisms with stains. However, a disadvantage to these automated systems is that it is extremely difficult to differentiate between the microorganisms with dust or scratches on blood agar plates because both the dust and scratches can create a highly diverse combination of shapes and appearances.[10]
Alternative units
Instead of colony-forming units, the parameters Most Probable Number (MPN) and Modified Fishman Units (MFU) can be used. The Most Probable Number method counts viable cells and is useful when enumerating low concentrations of cells or enumerating microbes in products where particulates make plate counting impractical.[11] Modified Fishman Units take into account bacteria which are viable, but non-culturable.
See also
- Cell counting
- Growth medium
- Miles and Misra method
- Most probable number
- Replica plating
- Viral plaque
References
- ↑ Goldman, Emanuel; Green, Lorrence H (24 August 2008). Practical Handbook of Microbiology, Second Edition (Google eBook) (Second ed.). USA: CRC Press, Taylor and Francis Group. p. 864. ISBN 978-0-8493-9365-5. Retrieved 2014-10-16.
- ↑ Breed RS, Dotterrer WD (May 1916). "The Number of Colonies Allowable on Satisfactory Agar Plates". Journal of Bacteriology 1 (3): 321–31. PMC 378655. PMID 16558698.
- 1 2 3 "USP 61: Microbial Enumeration Tests" (PDF). United States Pharmacopeia. Retrieved 24 March 2015.
- ↑ Reynolds, Jackie. "Serial Dilution Protocols". www.microbelibrary.org. Retrieved 2015-11-15.
- ↑ Geissmann Q (2013). "OpenCFU, a new free and open-source software to count cell colonies and other circular objects". PLoS ONE 8 (2): e54072. doi:10.1371/journal.pone.0054072. PMC 3574151. PMID 23457446.
- ↑ http://www.nist.gov/pml/div686/sources_detectors/nice.cfm[]
- ↑ Clarke ML, Burton RL, Hill AN, Litorja M, Nahm MH, Hwang J (August 2010). "Low-cost, high-throughput, automated counting of bacterial colonies". Cytometry Part A 77 (8): 790–7. doi:10.1002/cyto.a.20864. PMC 2909336. PMID 20140968.
- ↑ Cai Z, Chattopadhyay N, Liu WJ, Chan C, Pignol JP, Reilly RM (November 2011). "Optimized digital counting colonies of clonogenic assays using ImageJ software and customized macros: comparison with manual counting". International Journal of Radiation Biology 87 (11): 1135–46. doi:10.3109/09553002.2011.622033. PMID 21913819.
- ↑ Vokes MS, Carpenter AE (April 2008). "Using CellProfiler for automatic identification and measurement of biological objects in images". Current Protocols in Molecular Biology. Chapter 14: Unit 14.17. doi:10.1002/0471142727.mb1417s82. PMID 18425761.
- ↑ Brugger, Silvio D.; Baumberger, Christian; Jost, Marcel; Jenni, Werner; Brugger, Urs; Mühlemann, Kathrin (2012-03-20). "Automated Counting of Bacterial Colony Forming Units on Agar Plates". PLoS ONE 7 (3). doi:10.1371/journal.pone.0033695. ISSN 1932-6203. PMC 3308999. PMID 22448267.
- ↑ "Bacterial Analytical Manual: Most Probable Number from Serial Dilutions". United States Food and Drug Administration. October 2010.
Further reading
- Fishman, William H.; Bernfeld, Peter (1955). "[31] Glucuronidases". Methods in Enzymology 1: 262–9. doi:10.1016/0076-6879(55)01035-5. ISBN 978-0-12-181801-2.