Cockayne syndrome (CS), also called Neill-Dingwall syndrome, is a rare autosomal recessive neurodegenerative disorder characterized by growth failure, impaired development of the nervous system, abnormal sensitivity to sunlight (photosensitivity), eye disorders and premature aging.[1][2][3] Failure to thrive and neurological disorders are criteria for diagnosis, while photosensitivity, hearing loss, eye abnormalities, and cavities are other very common features.[3] Problems with any or all of the internal organs are possible. It is associated with a group of disorders called leukodystrophies, which are conditions characterized by degradation of neurological white matter. The underlying disorder is a defect in a DNA repair mechanism.[4] Unlike other defects of DNA repair, patients with CS are not predisposed to cancer or infection.[5] Cockayne syndrome is a rare but destructive disease usually resulting in death within the first or second decade of life. The mutation of specific genes in Cockayne syndrome is known, but the widespread effects and its relationship with DNA repair is yet to be well understood.[5]
It is named after English physician Edward Alfred Cockayne (1880–1956) who first described it in 1936 and re-described in 1946.[6] Neill-Dingwall syndrome was named after Mary M. Dingwall and Catherine A. Neill.[6] These women described the case of two brothers with Cockayne syndrome and asserted it was the same disease described by Cockayne. In their article the women contributed to the symptoms of the disease through their discovery of calcifications in the brain. They also compared Cockayne syndrome to what is now known as Hutchinson-Gilford progeria syndrome (HGPS), then called progeria, due to the advanced aging that characterizes both disorders.[6]
Forms
- CS Type I, the "classic" form, is characterized by normal fetal growth with the onset of abnormalities in the first two years of life. Vision and hearing gradually decline.[7] The central and peripheral nervous systems progressively degenerate until death in the first or second decade of life as a result of serious neurological degradation. Cortical atrophy is less severe in CS Type I.[8]
- CS Type II is present from birth (congenital) and is much more severe than CS Type 1.[7] It involves very little neurological development after birth. Death usually occurs by age seven. This specific type has also been designated as cerebro-oculo-facio-skeletal (COFS) syndrome or Pena-Shokeir syndrome Type II.[7] COFS syndrome is named so due to the effects it has on the brain, eyes, face, and skeletal system, as the disease frequently causes brain atrophy, cataracts, loss of fat in the face, and osteoporosis. COFS syndrome can be further subdivided into several conditions (COFS types 1, 2, 3 (associated with xeroderma pigmentosum) and 4).[9] Typically patients with this early-onset form of the disorder show more severe brain damage, including reduced myelination of white matter, and more widespread calcifications, including in the cortex and basal ganglia.[8]
- CS Type III, characterized by late onset, is typically milder than Types I and II.[7] Often patients with Type III will live into adulthood.
- Xeroderma pigmentosum-Cockayne syndrome (XP-CS) occurs when an individual also suffers from xeroderma pigmentosum, another DNA repair disease. Some symptoms of each disease are expressed. For instance, freckling and pigment abnormalities characteristic of XP are present. The neurological disorder, spasticity, and underdevelopment of sexual organs characteristic of CS are seen. However, hypomyelination and the facial features of typical CS patients are not present.[10]
Physical appearance and diagnosis
Persons with this syndrome have smaller than normal head sizes (microcephaly), are of short stature (dwarfism), their eyes appear sunken, and they have an "aged" look. They often have long limbs with joint contractures (inability to relax muscle at a joint), a hunched back (kyphosis), and they may be very thin (cachetic), due to a loss of subcutaneous fat. Their small chin, large ears, and pointy, thin nose often give an aged appearance.[8]
The skin of those with Cockayne syndrome is also frequently affected. Hyperpigmentation, varicose or spider veins (telangiectasia),[8] and serious sensitivity to sunlight are common, even in individuals without XP-CS. Often patients with Cockayne Syndrome will severely burn or blister with very little exposure.
The eyes of patients can be affected in various ways and eye abnormalities are common in CS. Cataracts and cloudiness of the cornea (corneal opacity) are common. The loss of and damage to nerves of the optic nerve, causing optic atrophy can occur.[3] Nystagmus, or involuntary eye movement, and pupils that fail to dilate demonstrate a loss of control of voluntary and involuntary muscle movement.[8] A salt and pepper retinal pigmentation is also a visible symptom.
Diagnosis is determined by a specific test for DNA repair, which measures the recovery of RNA after exposure to UV radiation. Despite being associated with genes involved in nucleotide excision repair (NER), unlike xeroderma pigmentosum, CS is not associated with an increased risk of cancer.[5]
Genetics
Cockayne syndrome is classified genetically as follows:
Mutations in the ERCC8 (also known as CSA) gene or the ERCC6 (also known as CSB) gene are the cause of Cockayne syndrome.[7] Mutations in the ERCC6 gene mutation makes up ~70% of cases. The proteins made by these genes are involved in repairing damaged DNA via the transcription-coupled repair mechanism, particularly the DNA in active genes. DNA damage is caused by ultraviolet rays from sunlight, radiation, or free radicals in the body. A normal cell can repair damage to DNA easily before it collects. If either the ERCC6 or the ERCC8 gene is altered (as in Cockayne Syndrome), DNA damage is not repaired. As this damage accumulates, it can lead to malfunctioning cells or cell death. This cell death and malfunctioning likely contributes to the symptoms of Cockayne Syndrome such as premature aging and hypomyelination of neurons.[7]
Neurology
Imaging studies reveal widespread absence of the myelin sheaths of the neurons in the white matter of the brain, and general atrophy of the cortex.[5] Calcifications have also been found in the putamen, an area of the forebrain that regulates movements and aids in some forms of learning,[8] along with in the cortex.[6] Additionally, atrophy of the central area of the cerebellum found in patients with Cockayne syndrome could also result in the lack of muscle control, particularly involuntary, and poor posture typically seen.
Treatment
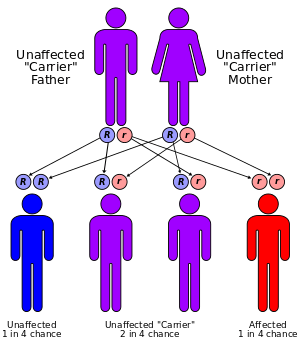
There is no permanent cure for this syndrome, although patients can be treated according to their specific symptoms. The prognosis for those with Cockayne syndrome is poor, as death typically occurs by the individual’s twenties. Treatment usually involves physical therapy and minor surgeries to the affected organs, like cataract removal.[3] Also wearing high-factor sunscreen and protective clothing is recommended as patients with Cockayne syndrome are very sensitive to UV radiation.[11] Optimal nutrition can also help. Genetic counseling for the parents is recommended, as the disorder has a 25% chance of being passed to any future children, and prenatal testing is also a possibility.[3] Another important aspect is prevention of recurrence of CS in other sibling. Identification of gene defects involved makes it possible to offer genetic counseling and antenatal
diagnostic testing to the parents who already have one affected child.[12]
See also
External links
References
- ↑ Bertola, Dr; Cao, H; Albano, Lm; Oliveira, Dp; Kok, F; Marques-Dias, Mj; Kim, Ca; Hegele, Ra (2006). "Cockayne syndrome type A: novel mutations in eight typical patients". Journal of Human Genetics 51 (8): 701–5. doi:10.1007/s10038-006-0011-7. PMID 16865293.
- ↑ James, William; Berger, Timothy; Elston, Dirk (2005). Andrews' Diseases of the Skin: Clinical Dermatology (10th ed.). Saunders. p. 575. ISBN 0-7216-2921-0.
- 1 2 3 4 5 Bender M, Potocki L, Metry D. What syndrome is this? Cockayne syndrome. Pediatric Dermatology [serial online]. November 2003;20(6):538-540. Available from: MEDLINE with Full Text, Ipswich, MA. Accessed April 30, 2015.
- ↑ Hoeijmakers JH (October 2009). "DNA damage, aging, and cancer". N. Engl. J. Med. 361 (15): 1475–85. doi:10.1056/NEJMra0804615. PMID 19812404.
- 1 2 3 4 Nance M, Berry S. Cockayne syndrome: review of 140 cases. American Journal of Medical Genetics. January 1, 1992;42(1):68-84. Available from: MEDLINE with Full Text, Ipswich, MA. Accessed April 30, 2015.
- 1 2 3 4 Neill CA, Dingwall MM. A Syndrome Resembling Progeria: A Review of Two Cases. Archives of Disease in Childhood. 1950;25(123):213-223.
- 1 2 3 4 5 6 Cockayne Syndrome. Genetics Home Reference http://ghr.nlm.nih.gov/condition/cockayne-syndrome Published April 28, 2015. Reviewed May 2010. Accessed April 30, 2015.
- 1 2 3 4 5 6 Javadzadeh M. Cockayne Syndrome. Iran J Child Neurol. Autumn 2014;8;4(Suppl.1):18-19.
- ↑ Cerebrooculofacioskeletal Syndrome 2. Online Mendelian Inheritance in Man. https://omim.org/entry/610756. Published 2/12/2007.
- ↑ Laugel V. Cockayne Syndrome. 2000 Dec 28 [Updated 2012 Jun 14]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015. Available from:
- ↑ Kyllermen, Marten. Cockayne Syndrome. Swedish Information Centre for Rare Diseases. 2012: 4.0. http://www.socialstyrelsen.se/rarediseases/cockaynesyndrome#anchor_17
- ↑ Title: Cockayne Syndrome Authors: Dr Nita R Sutay, Dr Md Ashfaque Tinmaswala, Dr Manjiri Karlekar, Dr Swati Jhahttp://jmscr.igmpublication.org/v3-i7/35%20jmscr.pdf
- ↑ http://www.orpha.net/consor/cgi-bin/OC_Exp.php?Lng=GB&Expert=1317
|
|---|
| | DNA repair | |
|---|
| | Lamin A/C | |
|---|
| | Other/Related disorders | |
|---|
| See also: DNA replication and repair-deficiency disorder |
|