Enoxaparin sodium
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Lovenox, Clexane, Oksapar, other |
| AHFS/Drugs.com | monograph |
| MedlinePlus | a696006 |
| Licence data | US FDA:link |
| Pregnancy category |
|
| Legal status | |
| Routes of administration | Subcutaneous (SC) Injection and intravenous (IV) per package insert |
| Pharmacokinetic data | |
| Biological half-life | 4.5 hours |
| Identifiers | |
| CAS Number |
9005-49-6 |
| ATC code | B01AB05 |
| PubChem | CID 772 |
| IUPHAR/BPS | 6811 |
| DrugBank |
DB01225 |
| ChemSpider | none |
| UNII |
8NZ41MIK1O |
| KEGG |
D07510 |
| ChEMBL |
CHEMBL1201685 |
| Chemical data | |
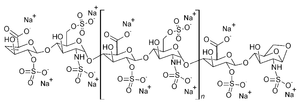
| Formula | (C26H40N2O36S5)n |
| Molar mass | 4500 g/mol (average) |
| | |
Enoxaparin sodium is a low molecular weight heparin marketed under the trade names Lovenox, Xaparin and Clexane, among others.[1] It is an anticoagulant used to prevent deep vein thrombosis (DVT) or to treat acute deep vein thrombosis with or without pulmonary embolism (PE).[2] It is given as an injection just under the skin or intravenously.[2] Its use is evolving in acute coronary syndromes (ACS).[3]
Enoxaparin is manufactured by Sanofi and is derived from the intestinal mucosa of pigs.[2] Generic versions are available from Amphastar Pharmaceuticals and Sandoz.[4]
It is on the WHO Model List of Essential Medicines, the most important medications needed in a basic health system.[5]
Medical uses
- Treatment of unstable angina (UA) and non-Q-wave myocardial infarction (NQMI), administered concurrently with aspirin
- DVT prophylaxis in knee replacement surgery
- DVT prophylaxis in hip replacement surgery
- DVT prophylaxis in abdominal surgery
- Treatment of DVT with or without pulmonary embolism
- Treatment of DVT inpatient, with ST-segment elevation myocardial infarction (STEMI)[2]
Monitoring
Enoxaparin has predictable absorption, bioavailability, and distribution therefore monitoring is not typically done. However, there are instances where monitoring may be beneficial for special populations, for example individuals with kidney insufficiency or those that are obese. In this case, anti-Xa units can be measured and dosing adjusted accordingly.[2]
Reversal agent
Protamine sulfate is less effective at reversing enoxaparin compared to heparin, with a maximum neutralization of approximately 60% of the anti-factor Xa effect.[2]
Pregnancy
- Enoxaparin is a FDA pregnancy category B drug which means enoxaparin is not expected to cause harm to an unborn baby when used during pregnancy. This statement is based on reproductive studies involving pregnant rats and rabbits. No birth defects or toxic effects to an unborn fetus due to enoxaparin were observed during these animals studies. However a human's response to enoxaparin might be different than that of a small animal, therefore enoxaparin should be used during pregnancy only if there is a definite need.[2]
- Enoxaparin does not cross the placenta therefore it is unlikely an unborn baby would be exposed to it.[2]
- Some fetal deaths have been reported by women who used enoxaparin during pregnancy, but it is unclear if enoxaparin caused these deaths.[2]
- Pregnant woman on enoxaparin should be monitored on a regular basis for bleeding and/or "excessive anticoagulation" especially when the delivery date is approaching. The risk of hemorrhage is higher during delivery if the person is still using enoxaparin and this could endanger the life of the baby and/or the mom.[2]
- The multiple-dose vials of the brand name enoxaparin (Lovenox) contain 15 mg benzyl alcohol per 1 mL as a preservative. Premature infants who have been given large amounts of benzyl alcohol (99-405 mg/kg/day) have experienced "gasping syndrome".[2][6]
- Although enoxaparin is used to prevent blood clots it is necessary to remember that pregnancy alone can raise a woman's risk of clotting.[2]
Side effects
Uncommon (<1%)
- In people with unstable angina or non-Q-wave myocardial infarction:
- Atrial fibrillation, heart failure, lung edema, pneumonia: ≥ 0.5%[2]
Common (>1%)
- Thrombocytopenia, i.e. can be associated with heparin-induced thrombocytopenia (0.5-5.0% of persons treated for at least five days)[7]
- Elevations in serum aminotransferases: 5.9%-6.1%[2]
- In people undergoing abdominal or colorectal surgery:
- Bleeding, anemia, ecchymosis: ≥ 2%[2]
- In persons undergoing hip or knee replacement:
- In persons with severely restricted mobility during acute illness:
- In people being treated for deep vein thrombosis:
Frequency under investigation
- Local reactions: local irritation, pain, hematoma, ecchymosis, erythema[2]
- Bleeding[2]
- Hyperkalemia[2]
- Transaminitis[2]
- Hemorrhage[8]
Boxed warning
The FDA issued a revision to the boxed warning for enoxaparin in October 2013.[8] The revision recommends exercising caution regarding when spinal catheters are placed and removed in persons taking enoxaparin for spinal puncture or neuroaxial anesthesia.[9] It may be necessary to delay anticoagulant dosing in these persons in order to decrease the risk for spinal or epidural hematomas, which can manifest as permanent or long-term paralysis.[9] Persons at risk for hematomas may present with indwelling epidural catheters, concurrent use of medications that worsen bleeding states such as non-steroidal anti-inflammatory drugs (NSAIDs), or a past medical history of epidural or spinal punctures, spinal injury, or spinal deformations.[8] The FDA recommends that at-risk persons be monitored for bleeding and neurological changes.[8][10]
Pharmacology
Mechanism of action
Enoxaparin binds to antithrombin (a circulating anticoagulant) to form a complex that irreversibly inactivates clotting factor Xa.[11] It has less activity against factor IIa (thrombin) compared to unfractionated heparin (UFH) due to its low molecular weight.[12]
Pharmacokinetics
Absorption: Bioavailability (subcutaneous injection) ~ 100%[8]
Distribution: Volume of distribution (anti-Factor Xa activity) = 4.3 liters[8]
Metabolism: Enoxaparin is metabolized in the liver into low molecular weight species by either or both desulfation and depolymerization.[8]
Elimination: A single dose of a subcutaneous injection of enoxaparin has an elimination half-life of 4.5 hours.[8] Approximately 10%-40% of the active and inactive fragments from a single dose are excreted by the kidneys.[8] Dose adjustments based on kidney function are necessary in persons with reduced kidney function.[8]
Drug class
Enoxaparin belongs to the class of drugs known as low molecular weight heparins. Other drugs in this class include dalteparin and tinzaparin.[13]
References
- ↑ Issa, Abdelfatah Abou; Simman, Richard (2015-03-18). "Lovenox Induced Tissue Necrosis, a Case Report and Literature Review". The Journal of the American College of Clinical Wound Specialists 5 (3): 66–68. doi:10.1016/j.jccw.2015.03.001. ISSN 2213-5103. PMC 4495755. PMID 26199895.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 "Lovenox® (enoxaparin sodium injection) for subcutaneous and intravenous use Prescribing Information". products.sanofi.us. Retrieved 2015-11-03.
- ↑ Lee, Sinjin; Gibson, C. Michael (2007-05-01). "Enoxaparin in acute coronary syndromes". Expert Review of Cardiovascular Therapy 5 (3): 387–399. doi:10.1586/14779072.5.3.387. ISSN 1744-8344. PMID 17489664.
- ↑ Mourier, Pierre A. J.; Agut, Christophe; Souaifi-Amara, Hajer; Herman, Fréderic; Viskov, Christian (2015-11-10). "Analytical and statistical comparability of generic enoxaparins from the US market with the originator product". Journal of Pharmaceutical and Biomedical Analysis 115: 431–442. doi:10.1016/j.jpba.2015.07.038. ISSN 1873-264X. PMID 26280926.
- ↑ "19th WHO Model List of Essential Medicines (April 2015)" (PDF). WHO. April 2015. Retrieved May 10, 2015.
- ↑ Gershanik, Juan; Boecler, Betty; Ensley, Harry; McCloskey, Sharon; George, William (1982-11-25). "The Gasping Syndrome and Benzyl Alcohol Poisoning". New England Journal of Medicine 307 (22): 1384–1388. doi:10.1056/NEJM198211253072206. ISSN 0028-4793. PMID 7133084.
- ↑ "Reducing Thrombotic Complications in the Perioperative Setti... : Anesthesia & Analgesia" (PDF). LWW. Retrieved 2015-11-05.
- 1 2 3 4 5 6 7 8 9 10 "DailyMed - ENOXAPARIN SODIUM- enoxaparin sodium injection". dailymed.nlm.nih.gov. Retrieved 2015-11-05.
- 1 2 Research, Center for Drug Evaluation and. "Drug Safety and Availability - FDA Drug Safety Communication: Updated recommendations to decrease risk of spinal column bleeding and paralysis in patients on low molecular weight heparins". www.fda.gov. Retrieved 2015-11-05.
- ↑ Commissioner, Office of the. "Safety Information - Lovenox (enoxaparin sodium) injection". www.fda.gov. Retrieved 2015-11-05.
- ↑ Alldredge, Brian; Corelli, Robin; Ernst, Michael; Guglielmo, B. Joseph; Jacobson, Pamela; Kradjan, Wayne; Williams, Bradley. Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs (Tenth ed.). pp. 347–348. ISBN 1-60913-713-2.
- ↑ Trevor, Anthony J., Bertram G. Katzung, and Susan B. Masters. Basic & clinical pharmacology. McGraw-Hill Medical, 2012.
- ↑ "LowMolecularWeightHeparins". livertox.nih.gov. Retrieved 2015-11-05.
External links
- Official website
- DrugBank Information
- Lovenox: Prescribing Information
- "Enoxaparin Sodium". Daily Med.
| ||||||||||||||||||||||||||||||||||||||||||