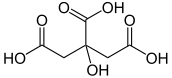
Citric acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3-carboxy-3-hydroxypentane-1,5-dioic acid | |||
| Other names | |||
| Identifiers | |||
| 77-92-9 | |||
| ChEBI | CHEBI:30769 | ||
| ChEMBL | ChEMBL1261 | ||
| ChemSpider | 305 | ||
| DrugBank | DB04272 | ||
| EC Number | 201-069-1 | ||
| 2478 | |||
| Jmol interactive 3D | Image | ||
| KEGG | D00037 | ||
| PubChem | 311 | ||
| RTECS number | GE7350000 | ||
| UNII | XF417D3PSL | ||
| |||
| |||
| Properties | |||
| C6H8O7 | |||
| Molar mass | 192.12 g·mol−1 | ||
| Appearance | crystalline white solid | ||
| Odor | odorless | ||
| Density | 1.665 g/cm3 (anhydrous) 1.542 g/cm3 (18 °C, monohydrate) | ||
| Melting point | 156 °C (313 °F; 429 K) | ||
| Boiling point | 310 °C (590 °F; 583 K) decomposes from 175 °C[2] | ||
| 117.43 g/100 mL (10 °C) 147.76 g/100 mL (20 °C) 180.89 g/100 mL (30 °C) 220.19 g/100 mL (40 °C) 382.48 g/100 mL (80 °C) 547.79 g/100 mL (100 °C)[3] | |||
| Solubility | soluble in alcohol, ether, ethyl acetate, DMSO insoluble in C6H6, CHCl3, CS2, toluene[2] | ||
| Solubility in ethanol | 62 g/100 g (25 °C)[2] | ||
| Solubility in amyl acetate | 4.41 g/100 g (25 °C)[2] | ||
| Solubility in diethyl ether | 1.05 g/100 g (25 °C)[2] | ||
| Solubility in 1,4-Dioxane | 35.9 g/100 g (25 °C)[2] | ||
| log P | -1.64 | ||
| Acidity (pKa) | pKa1 = 3.13[4] pKa2 = 4.76[4] pKa3 = 6.39,[5] 6.40[6] | ||
| Refractive index (nD) |
1.493 - 1.509 (20 °C)[3] 1.46 (150 °C)[2] | ||
| Viscosity | 6.5 cP (50% aq. sol.)[3] | ||
| Structure | |||
| Monoclinic | |||
| Thermochemistry | |||
| 226.51 J/mol·K (26.85 °C)[7] | |||
| Std molar entropy (S |
252.1 J/mol·K[7] | ||
| Std enthalpy of formation (ΔfH |
-1548.8 kJ/mol[3] | ||
| Std enthalpy of combustion (ΔcH |
-1960.6 kJ/mol[7] -1972.34 kJ/mol (monohydrate)[3] | ||
| Pharmacology | |||
| ATC code | A09 | ||
| Hazards | |||
| Main hazards | skin and eye irritant | ||
| Safety data sheet | HMDB | ||
| GHS pictograms |  [4] [4] | ||
| GHS signal word | Warning | ||
| H319[4] | |||
| P305+351+338[4] | |||
| EU classification (DSD) |
| ||
| R-phrases | R34, R36/37/38, R41 | ||
| S-phrases | S24/25, S26, S36/37/39, S45 | ||
| NFPA 704 | |||
| Flash point | 155 °C (311 °F; 428 K) | ||
| 345 °C (653 °F; 618 K) | |||
| Explosive limits | 8%[4] | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (Median dose) |
3000 mg/kg (rats, oral) | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Citric acid is a weak organic tribasic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms.
More than a million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and as a chelating agent.[8]
Natural occurrence and industrial production

Citric acid exists in greater than trace amounts in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L in the juices[9]).[lower-alpha 1] The concentrations of citric acid in citrus fruits range from 0.005 mol/L for oranges and grapefruits to 0.30 mol/L in lemons and limes. Within species, these values vary depending on the cultivar and the circumstances in which the fruit was grown.
Industrial-scale citric acid production first began in 1890 based on the Italian citrus fruit industry, where the juice was treated with hydrated lime (calcium hydroxide) to precipitate calcium citrate, which was isolated and converted back to the acid using diluted sulfuric acid.[10] In 1893, C. Wehmer discovered Penicillium mold could produce citric acid from sugar. However, microbial production of citric acid did not become industrially important until World War I disrupted Italian citrus exports.
In 1917, American food chemist James Currie discovered certain strains of the mold Aspergillus niger could be efficient citric acid producers, and the pharmaceutical company Pfizer began industrial-level production using this technique two years later, followed by Citrique Belge in 1929. In this production technique, which is still the major industrial route to citric acid used today, cultures of A. niger are fed on a sucrose or glucose-containing medium to produce citric acid. The source of sugar is corn steep liquor, molasses, hydrolyzed corn starch or other inexpensive sugary solutions.[11] After the mold is filtered out of the resulting solution, citric acid is isolated by precipitating it with calcium hydroxide to yield calcium citrate salt, from which citric acid is regenerated by treatment with sulfuric acid, as in the direct extraction from citrus fruit juice.
In 1977, a patent was granted to Lever Brothers for the chemical synthesis of citric acid starting either from aconitic or isocitrate/alloisocitrate calcium salts under high pressure conditions. This produced citric acid in near quantitative conversion under what appeared to be a reverse non-enzymatic Krebs cycle reaction.[12]
In 2007, worldwide annual production stood at approximately 1,600,000 tons.[13] More than 50% of this volume was produced in China. More than 50% was used as acidity regulator in beverages, some 20% in other food applications, 20% for detergent applications and 10% for related applications other than food, such as cosmetics, pharmaceutics and in the chemical industry.
Chemical characteristics

Citric acid was first isolated in 1784 by the chemist Carl Wilhelm Scheele, who crystallized it from lemon juice.[10][14] It can exist either in an anhydrous (water-free) form or as a monohydrate.The anhydrous form crystallizes from hot water, while the monohydrate forms when citric acid is crystallized from cold water. The monohydrate can be converted to the anhydrous form at c. 78 °C. Citric acid also dissolves in absolute (anhydrous) ethanol (76 parts of citric acid per 100 parts of ethanol) at 15 °C. It decomposes with loss of carbon dioxide above ca. 175 °C.
Citric acid is normally considered to be a tribasic acid, with pK values, extrapolated to zero ionic strength, of 5.21, 4.28 and 2.92 at 25C.[15] The pKa of the hydroxyl group has been found, by means of 13C NMR spectroscopy, to be 14.4.[16] The speciation diagram shows that solutions of citric acid are buffer solutions between about pH 2 and pH 8. In biological systems around pH 7, the two species present are the citrate ion and mono-hydrogen citrate ion. The SSC 20X hybridization buffer is an example in common use.[17] Tables compiled for biochemical studies[18] are available.
On the other hand, the pH of a 1mM solution of citric acid will be about 3.2. The pH of fruit juices from citrus fruits like oranges and lemons depends on the citric acid concentration, being lower the higher the acid concentration and vice versa.
Acid salts of citric acid can be prepared by careful adjustment of the pH before crystallizing the compound. See, for example, sodium citrate.
The citrate ion forms complexes with metallic cations. The stability constants for the formation of these complexes are quite large because of the chelate effect. Consequently, it forms complexes even with alkali metal cations. However, when a chelate complex is formed using all three carboxylate groups the chelate rings have 7- and 8-members, which are generally less stable, thermodymically, than smaller chelate rings. In consequence the hydroxyl group can be deprotonated, forming part of a more stable 5-membered ring, as in ammonium ferric citrate,(NH4)5Fe(C6H4O7)2·2H2O.[19]
Esters such as triethyl citrate can be made.
Biochemistry
Citric acid cycle
Citrate is an intermediate in the TCA cycle (aka TriCarboxylic Acid cycle, Krebs cycle, Szent-Györgyi — Krebs cycle), a central metabolic pathway for animals, plants and bacteria. Citrate synthase catalyzes the condensation of oxaloacetate with acetyl CoA to form citrate. Citrate then acts as the substrate for aconitase and is converted into aconitic acid. The cycle ends with regeneration of oxaloacetate. This series of chemical reactions is the source of two-thirds of the food-derived energy in higher organisms. Hans Adolf Krebs received the 1953 Nobel Prize in Physiology or Medicine for the discovery.
Some bacteria, notably E. coli, can produce and consume citrate internally as part of their TCA cycle, but are unable to use it as food because they lack the enzymes required to import it into the cell. The acquisition by these bacteria, after tens of thousands of generations, of the ability to use citrate as food was studied by Lenski et al.[20][21] to explore mechanisms of evolution under selective pressure (in this case, a citrate-containing culture medium with limited amounts of other foods). They found evidence that in this case the innovation occurred via an accumulation of several somewhat rare mutations, none of which by itself would confer the selective advantage, rather than by a single extremely rare mutation.
Other biological roles
Citrate can be transported out of the mitochondria and into the cytoplasm, then broken down into acetyl-CoA for fatty acid synthesis and into oxaloacetate. Citrate is a positive modulator of this conversion, and allosterically regulates the enzyme acetyl-CoA carboxylase, which is the regulating enzyme in the conversion of acetyl-CoA into malonyl-CoA (the commitment step in fatty acid synthesis). In short, citrate is transported to the cytoplasm, converted to acetyl CoA, which is converted into malonyl CoA by the acetyl CoA carboxylase, which is allosterically modulated by citrate.
High concentrations of cytosolic citrate can inhibit phosphofructokinase, the catalyst of one of the rate-limiting steps of glycolysis. This effect is advantageous: high concentrations of citrate indicate that there is a large supply of biosynthetic precursor molecules, so there is no need for phosphofructokinase to continue to send molecules of its substrate, fructose 6-phosphate, into glycolysis. Citrate acts by augmenting the inhibitory effect of high concentrations of ATP, another sign that there is no need to carry out glycolysis.[22]
Citrate is a vital component of bone, helping to regulate the size of apatite crystals.[23]
Applications
Food and drink

Because it is one of the stronger edible acids, the dominant use of citric acid is as a flavoring and preservative in food and beverages, especially soft drinks.[10] Within the European Union it is denoted by E number E330. Citrate salts of various metals are used to deliver those minerals in a biologically available form in many dietary supplements. The buffering properties of citrates are used to control pH in household cleaners and pharmaceuticals. In the United States the purity requirements for citric acid as a food additive are defined by the Food Chemicals Codex, which is published by the United States Pharmacopoeia (USP).
Citric acid can be added to ice cream as an emulsifying agent to keep fats from separating, to caramel to prevent sucrose crystallization, or in recipes in place of fresh lemon juice. Citric acid is used with sodium bicarbonate in a wide range of effervescent formulae, both for ingestion (e.g., powders and tablets) and for personal care (e.g., bath salts, bath bombs, and cleaning of grease). Citric acid sold in a dry powdered form is commonly sold in markets and groceries as "sour salt", due to its physical resemblance to table salt. It has use in culinary applications, as an alternative to vinegar or lemon juice, where a pure acid is needed.
Citric acid can be used in food coloring to balance the pH level of a normally basic dye.
Cleaning and chelating agent
Citric acid is an excellent chelating agent, binding metals. It is used to remove limescale from boilers and evaporators.[10] It can be used to soften water, which makes it useful in soaps and laundry detergents. By chelating the metals in hard water, it lets these cleaners produce foam and work better without need for water softening. Citric acid is the active ingredient in some bathroom and kitchen cleaning solutions. A solution with a 6% concentration of citric acid will remove hard water stains from glass without scrubbing. In the industry, it is used to dissolve rust from steel. Citric acid can be used in shampoo to wash out wax and coloring from the hair.
Illustrative of its chelating abilities, citric acid was the first successful eluant used for total ion-exchange separation of the lanthanides, during the Manhattan Project in the 1940s. In the 1950s, it was replaced by the far more efficient EDTA.
Cosmetics and pharmaceuticals
Citric acid is widely used as a pH adjusting agent in creams and gels of all kinds. In this role, it is classified in most jurisdictions as a processing aid and so does not need to be listed on ingredient lists.
Citric acid is an alpha hydroxy acid and used as an active ingredient in chemical peels.
Citric acid is commonly used as a buffer to increase the solubility of brown heroin. Single-use citric acid sachets have been used as an inducement to get heroin users to exchange their dirty needles for clean needles in an attempt to decrease the spread of HIV and hepatitis.[24] Other acidifiers used for brown heroin are ascorbic acid, acetic acid, and lactic acid; in their absence, a drug user will often substitute lemon juice or vinegar.
Citric acid is used as one of the active ingredients in the production of antiviral tissues.[25]
Other uses
Citric acid is used as an odorless alternative to white vinegar for home dyeing with acid dyes.
Sodium citrate is component of Benedict's reagent, used for identification both qualitatively and quantitatively, of reducing sugars.
Citric acid can be used as an alternative to nitric acid in passivation of stainless steel.[26]
Citric acid can be used as a lower-odor stop bath as part of the process for developing photographic film. Photographic developers are alkaline, so a mild acid is used to neutralize and stop their action quickly, but commonly used acetic acid leaves a strong vinegar odor in the darkroom.[27]
Citric acid/potassium-sodium citrate can be used as a blood acid regulator.
Synthesize solid materials from small molecules
In materials science, the Citrate-gel method is process similar to the sol-gel method, which is a method for producing solid materials from small molecules. During the synthetic process, metal salts or alkoxides are introduced into a citric acid solution. The formation of citric complexes is believed to balance the difference in individual behaviour of ions in solution, which results in a better distribution of ions and prevents the separation of components at later process stages. The polycondensation of ethylene glycol and citric acid starts above 100ºС, resulting in polymer citrate gel formation.
Safety
Although a weak acid, exposure to pure citric acid can cause adverse effects. Inhalation may cause cough, shortness of breath, or sore throat. Overingestion may cause abdominal pain and sore throat. Exposure of concentrated solutions to skin and eyes can cause redness and pain.[28] Long-term or repeated consumption may cause erosion of tooth enamel.[28][29][30]
Compendial status
- British Pharmacopoeia[31]
- Japanese Pharmacopoeia[32]
See also
| Wikimedia Commons has media related to Citric acid. |
- The closely related acids isocitric acid, aconitic acid, and propane-1,2,3-tricarboxylic acid (tricarballylic acid, carballylic acid)
- Acids in wine
References
- ↑ David R. Lide, ed. (2005). "Physical Constants of Organic Compounds". CRC Handbook of Chemistry and Physics (Internet Version ed.). Boca Raton, FL: CRC Press.
- 1 2 3 4 5 6 7 "citric acid". chemister.ru.
- 1 2 3 4 5 CID 311 from PubChem
- 1 2 3 4 5 6 Sigma-Aldrich Co., Citric acid. Retrieved on 2014-06-02.
- ↑ "Data for Biochemical Research". ZirChrom Separations, Inc. Retrieved January 11, 2012.
- ↑ "Ionization Constants of Organic Acids". Michigan State University. Retrieved January 11, 2012.
- 1 2 3 Citric acid in Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2014-06-02)
- ↑ Apleblat, Alexander (2014). Citric acid. Springer. ISBN 978-3-319-11232-9.
- ↑ Penniston KL, Nakada SY, Holmes RP, Assimos DG; Nakada; Holmes; Assimos (2008). "Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products" (PDF). Journal of Endourology 22 (3): 567–570. doi:10.1089/end.2007.0304. PMC 2637791. PMID 18290732.
- 1 2 3 4 Frank H. Verhoff (2005), "Citric Acid", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH
- ↑ Lotfy, Walid A.; Ghanem, Khaled M.; El-Helow, Ehab R. (2007). "Citric acid production by a novel Aspergillus niger isolate: II. Optimization of process parameters through statistical experimental designs". Bioresource Technology 98 (18): 3470–3477. doi:10.1016/j.biortech.2006.11.032. PMID 17317159.
- ↑ US 4056567-V.Lamberti and E.Gutierrez
- ↑ Berovic, M.; Legisa, M. (2007). "Citric acid production". Biotechnology Annual Review Volume 13. Biotechnology Annual Review 13. pp. 303–343. doi:10.1016/S1387-2656(07)13011-8. ISBN 9780444530325. PMID 17875481.
- ↑ Graham, Thomas (1842). Elements of chemistry, including the applications of the science in the arts. Hippolyte Baillière, foreign bookseller to the Royal College of Surgeons, and to the Royal Society, 219, Regent Street. p. 944. Retrieved June 4, 2010.
- ↑ Goldberg, Robert N; Kishore, Nand; Lennen, Rebecca M (2002). "Thermodynamic Quantities for the Ionization Reactions of Buffers". J.Phys.Chem.Ref.Data 31 (1): 231–370. doi:10.1063/1.1416902.
- ↑ Silva, Andre M. N.; Kong, Xiaole; Hider, Robert C. (2009). "Determination of the pKa value of the hydroxyl group in the α-hydroxycarboxylates citrate, malate and lactate by 13C NMR: implications for metal coordination in biological systems". Biometals 22 (5): 771–778. doi:10.1007/s10534-009-9224-5.
- ↑ Maniatis, T.; Fritsch, E. F.; Sambrook, J. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- ↑ Gomori, G. (1955). "16 Preparation of buffers for use in enzyme studies". Methods in Enzymology Volume 1. Methods in Enzymology 1. p. 138. doi:10.1016/0076-6879(55)01020-3. ISBN 9780121818012.
- ↑ Matzapetakis, M.; Raptopoulou, C. P.; Tsohos, A.; Papaefthymiou, V.; Moon, S.N.; Salifoglou, A. (1998). "Synthesis, Spectroscopic and Structural Characterization of the First Mononuclear, Water Soluble Iron−Citrate Complex, (NH4)5Fe(C6H4O7)2·2H2O". J. Am. Chem. Soc. 120 (50): 13266–13267. doi:10.1021/ja9807035.
- ↑ http://phys.org/news/2014-02-bacteria-freezer-yield-startling-results.html
- ↑ http://www.rpgroup.caltech.edu/courses/Evolution_GIST_2013/files_2013/articles/PNAS-2008-Blount-7899-906.pdf
- ↑ Stryer, Lubert; Berg, Jeremy; Tymoczko, John (2003). "Section 16.2: The Glycolytic Pathway Is Tightly Controlled". Biochemistry (5. ed., international ed., 3. printing ed.). New York: Freeman. ISBN 978-0716746843.
- ↑ Hu, Y.-Y.; Rawal, A.; Schmidt-Rohr, K. (December 2010). "Strongly bound citrate stabilizes the apatite nanocrystals in bone". Proceedings of the National Academy of Sciences 107 (52): 22425–22429. Bibcode:2010PNAS..10722425H. doi:10.1073/pnas.1009219107. PMC 3012505. PMID 21127269. Retrieved July 28, 2012.
- ↑ Garden, J., Roberts, K., Taylor, A., and Robinson, D. (2003). "Evaluation of the Provision of Single Use Citric Acid Sachets to Injecting Drug Users" (pdf). Scottish Center for Infection and Environmental Health.
- ↑ "Tissues that fight germs". CNN. July 14, 2004. Retrieved May 8, 2008.
- ↑ "Pickling and Passivating Stainless Steel" (PDF). Euro-inox.org. Retrieved 2013-01-01.
- ↑ Anchell, Steve. "The Darkroom Cookbook: 3rd Edition (Paperback)". Focal Press. Retrieved 2013-01-01.
- 1 2 "Citric acid". International Chemical Safety Cards. NIOSH.
- ↑ J. Zheng, F. Xiao, L.M. Qian, Z.R. Zhou; Xiao; Qian; Zhou (December 2009). "Erosion behavior of human tooth enamel in citric acid solution". Tribology International 42 (11–12): 1558–1564. doi:10.1016/j.triboint.2008.12.008.
- ↑ "Effect of Citric Acid on Tooth Enamel".
- ↑ British Pharmacopoeia Commission Secretariat (2009). "Index, BP 2009" (PDF). Retrieved February 4, 2010.
- ↑ "Japanese Pharmacopoeia, Fifteenth Edition" (PDF). 2006. Retrieved 4 February 2010.
- ↑ This still does not make the lemon particularly strongly acidic. This is because, as a weak acid, most of the acid molecules are not dissociated so not contributing to acidity inside the lemon or its juice.
| ||||||||||
|