Ciraparantag
 | |
| Systematic (IUPAC) name | |
|---|---|
|
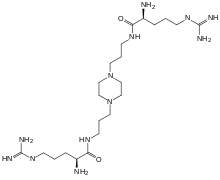
N,N'-(1,4-piperazinediyldi-3,1-propanediyl)bis[2-amino-5-[(aminoiminomethyl)amino]-, (2S,2'S)-pentanamide | |
| Clinical data | |
| Legal status |
|
| Routes of administration | Intravenous |
| Pharmacokinetic data | |
| Onset of action | 10 min |
| Duration of action | 24 hrs |
| Identifiers | |
| CAS Number | 1438492-26-2 |
| ATC code | None |
| PubChem | CID 71576543 |
| ChemSpider | 33427375 |
| UNII | U2R67KV65Q |
| Synonyms | PER977 |
| Chemical data | |
| Formula | C22H48N12O2 |
| Molar mass | 512.71 g·mol−1 |
| |
| |
Ciraparantag (INN/USAN, or aripazine) is a drug under investigation as an antidote for a number of anticoagulant (anti–blood clotting) drugs, including factor Xa inhibitors (rivaroxaban, apixaban and edoxaban), dabigatran, low molecular weight heparins and unfractionated heparin.[1][2]
Mechanism of action
According to in vitro studies, the substance binds directly to anticoagulants via hydrogen bonds from or to various parts of the molecule:[1]
| Hydrogen bonds | Rivaroxaban | Apixaban | Edoxaban | Dabigatran | Heparins |
|---|---|---|---|---|---|
| Guanidine part | |
|
|
| |
| α-Amino group | |
|
|
| |
| Amide nitrogen | |
|
| ||
| Amide oxygen | |
|
Chemical properties
Ciraparantag consists of two L-arginine units connected with a piperazine containing linker chain.[1]
See also
Other anticoagulant antidotes
References
This article is issued from Wikipedia - version of the Tuesday, November 10, 2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.